Show filters
Chemistry: Ten things we need to know about ice and snow
Understanding the molecular behaviour of frozen water is essential for predicting the future of our planet, says Thorsten Bartels-Rausch.
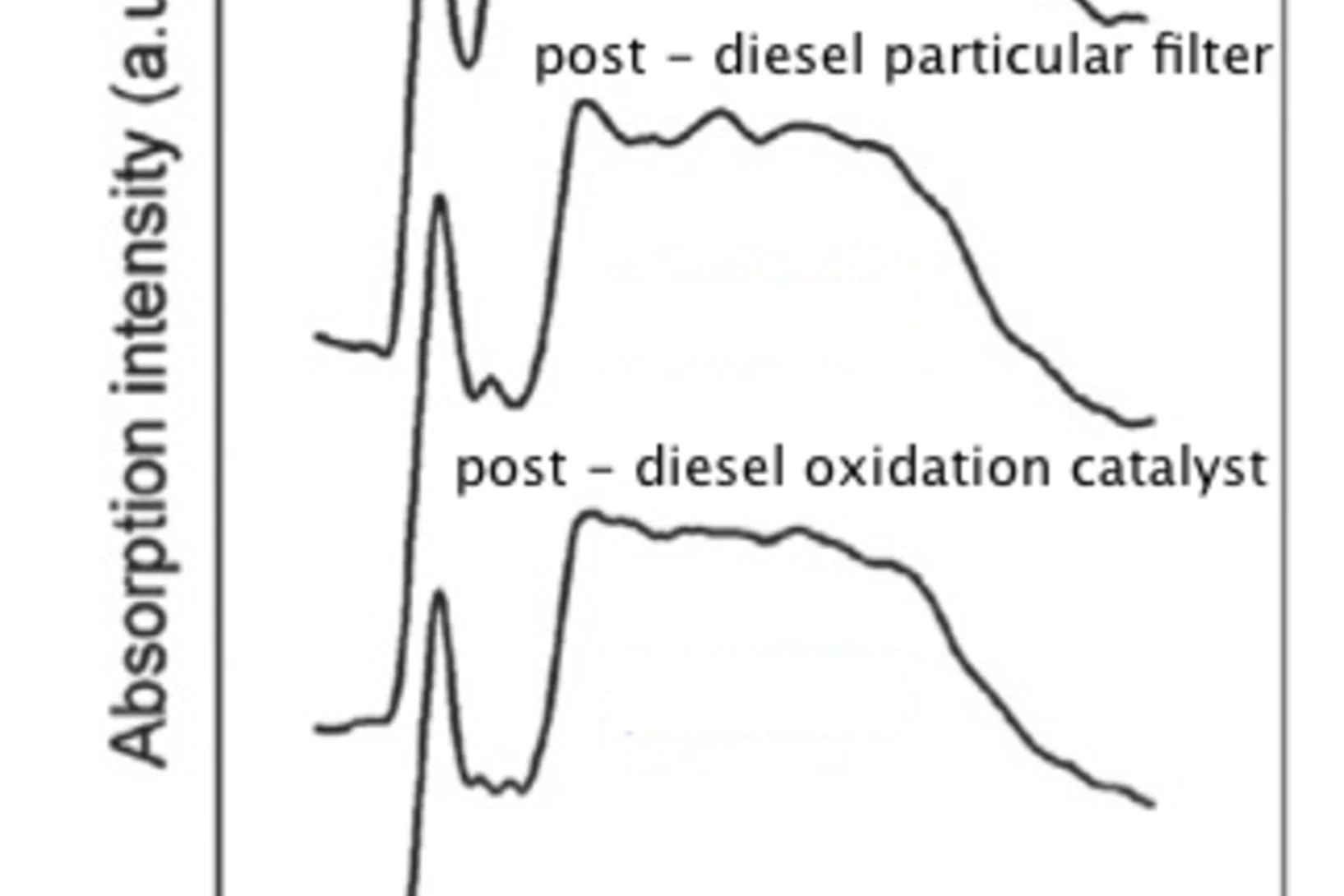
Variations in diesel soot reactivity along the exhaust after-treatment system, based on the morphology and nanostructure of primary soot particles
The reactivity of soot at different sites of the exhaust after-treatment system of a diesel engine (upstream and downstream of the diesel oxidation catalyst (DOC), downstream of the diesel particulate filter (DPF), as well as inside the DPF) was investigated on the basis of morphology and structure of primary soot particles by high resolution transmission electron microscopy (HRTEM). The results indicate that combustion-formed soot particles are susceptible to further transformations of their morphology within the exhaust system.
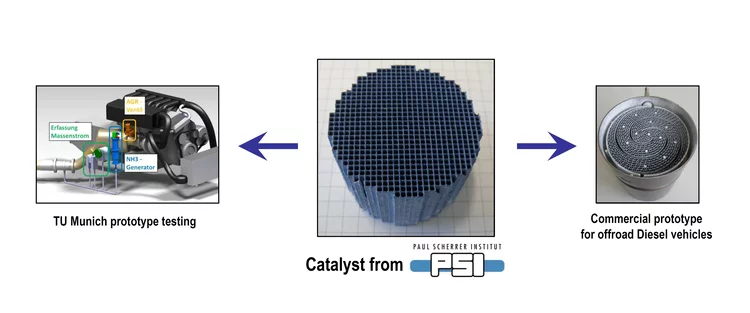
Supported gold as catalyst for the decomposition of ammonia precursors in the selective catalytic reduction of NOx
Titaniumdioxide supported gold was found to catalyze the hydrolysis of formate-based ammonia precursor compounds which are proposed for the selective catalytic reduction of nitrogen oxides (NOx) in combustion exhaust gas. In contrast to other noble metals, the supported gold does not oxidize the released NH3, while it maintains decomposition of intermediate formic acid.
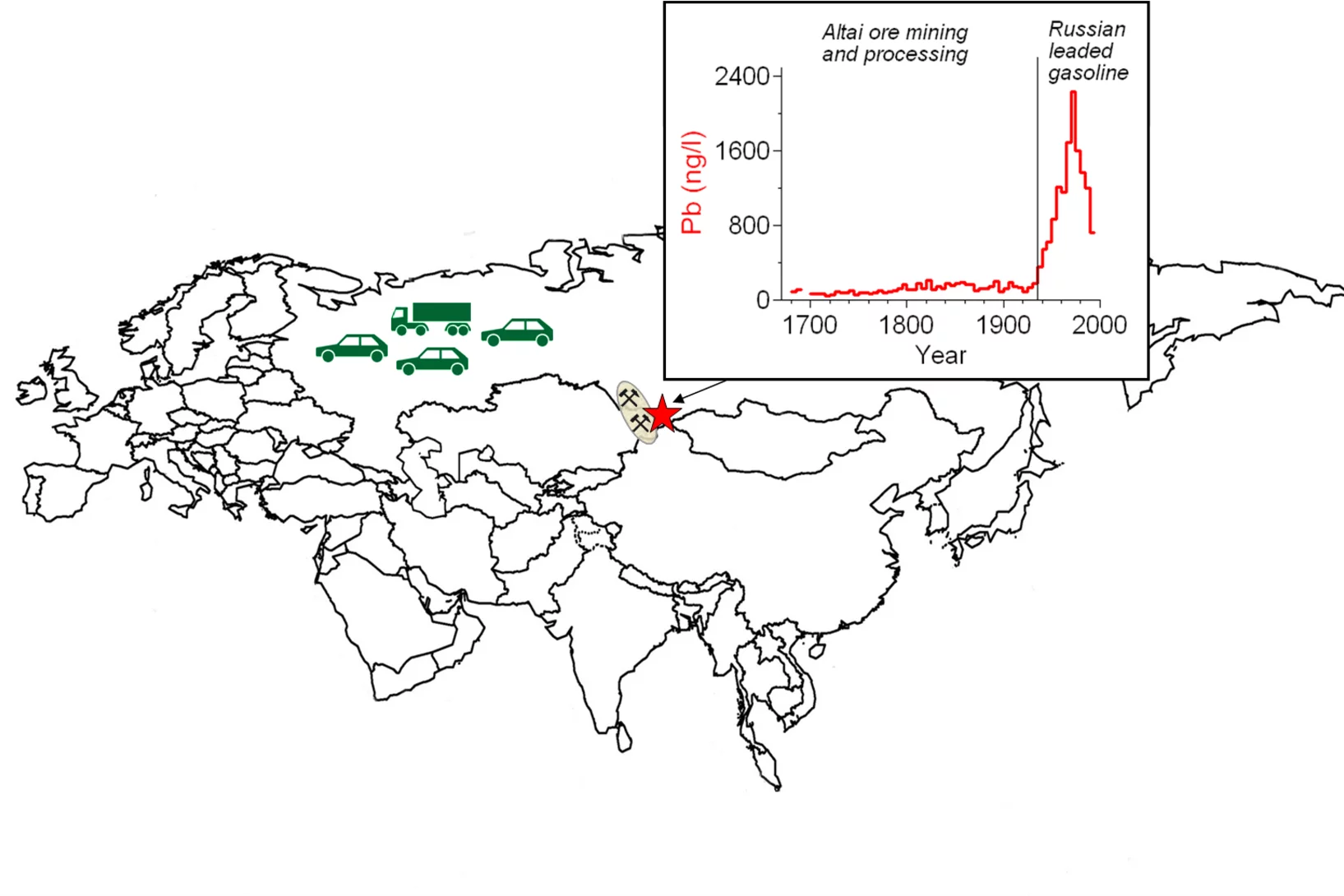
Three centuries of eastern european and Altai lead emissions recorded in a belukha ice core
Human activities have significantly altered atmospheric Pb concentrations and thus, its geochemical cycle, for thousands of years. Whereas historical Pb emissions from Western Europe, North America, and Asia are well documented, there is no equivalent data for Eastern Europe. Here, we present ice-core Pb concentrations for the period 1680à1995 from Belukha glacier in the Siberian Altai, assumed to be representative of emissions in Eastern Europe and the Altai.
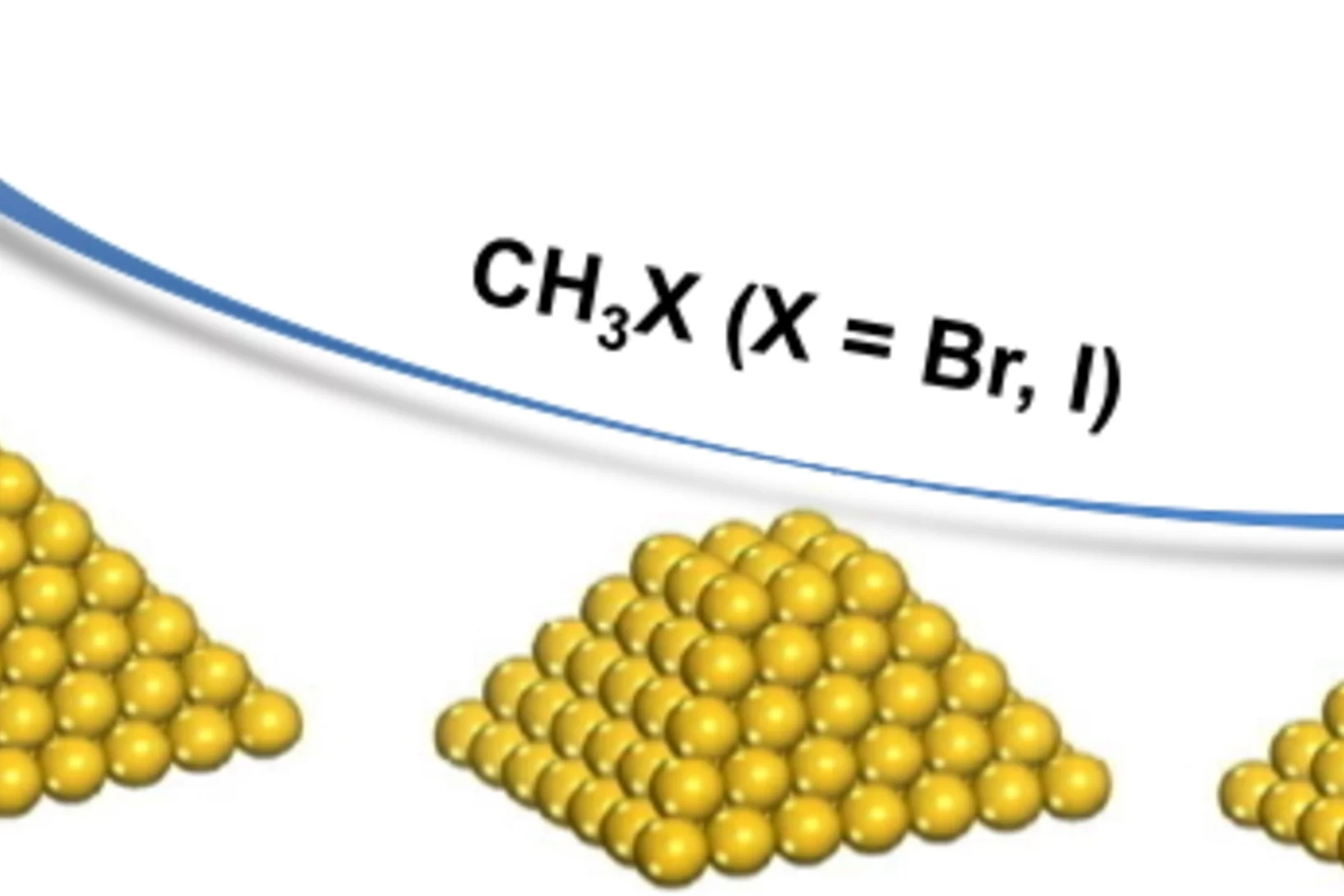
Influence of Methyl Halide Treatment on Gold Nanoparticles Supported on Activated Carbon
Gold particles supported on carbon when subjected to a flow of methyl iodide or bromide redisperse from large ensembles to single atoms and/or dimers of gold. Methyl halide oxidizes gold leading to gradual particle dissolution. The process could be carried out at temperatures as low as 50 °C. The excess of halide could be removed by a post-treatment of the material with 1%H2O/H2, which does not influence the metal dispersion. This remarkable transformation opens the possibility of re-activating gold catalysts that lost their performance due to metal particles sintering.
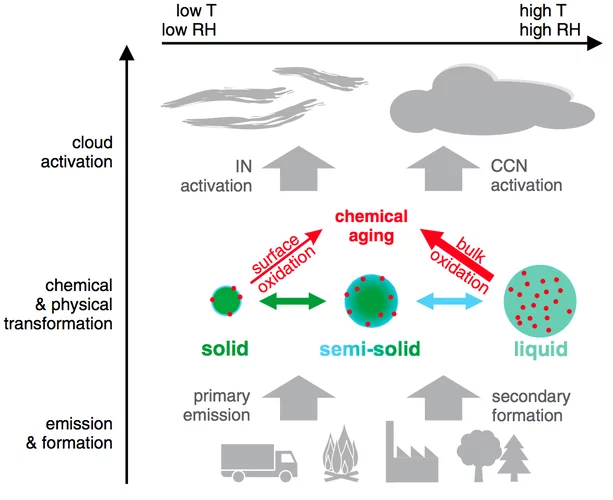
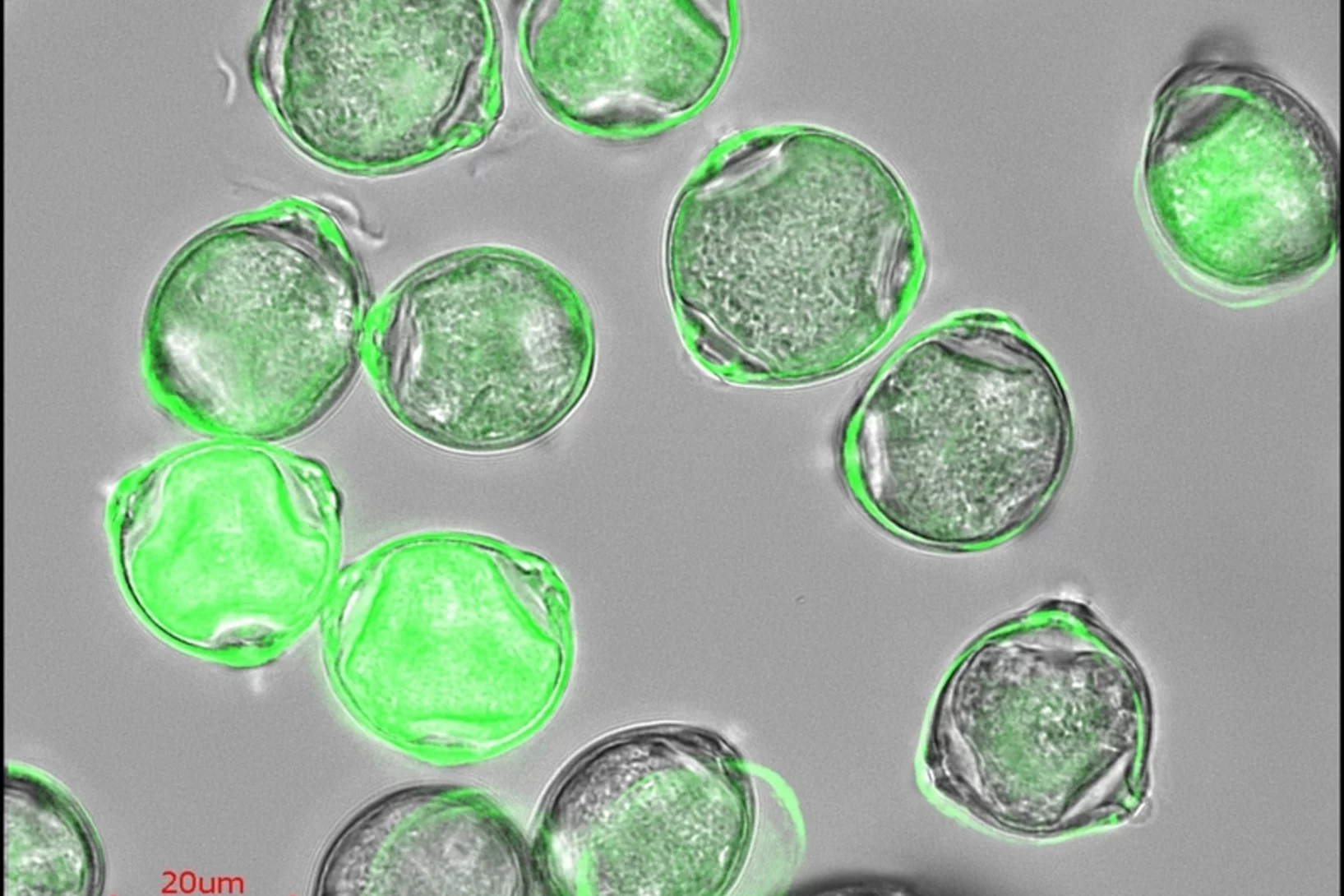
Gas uptake and chemical aging of semisolid organic aerosol particles
Organic substances can adopt an amorphous solid or semisolid state, influencing the rate of heterogeneous reactions and multiphase processes in atmospheric aerosols. Here we demonstrate how molecular diffusion in the condensed phase affects the gas uptake and chemical transformation of semisolid organic particles. Flow tube experiments show that the ozone uptake and oxidative aging of amorphous protein is kinetically limited by bulk diffusion.
The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles
The heterogeneous reactions of ozone with aerosol particles are of central importance to air quality. They are studied extensively, but the molecular mechanisms and kinetics remain unresolved. Based on new experimental data and calculations, we show that long-lived reactive oxygen intermediates (ROIs) are formed. The chemical lifetime of these intermediates exceeds 100 seconds, which is much longer than the surface residence time of molecular ozone (~ ns).
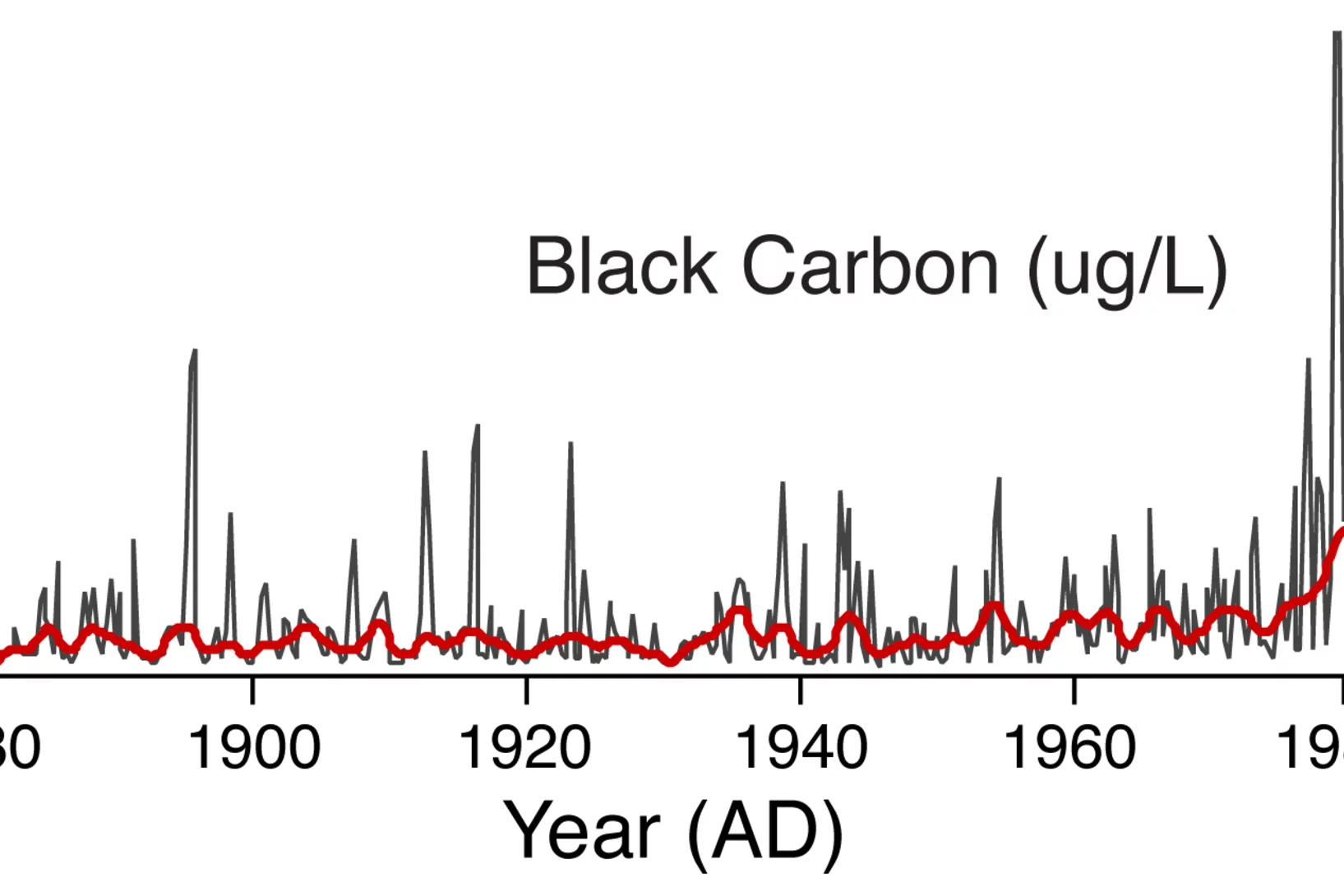
Recent increase in black carbon concentrations from a Mt. Everest ice core spanning 1860–2000 AD
A Mt. Everest ice core spanning 1860à2000 AD and analyzed at high resolution for black carbon (BC) using a Single Particle Soot Photometer demonstrates strong seasonality, with peak concentrations during the winter‐spring, and low concentrations during the summer monsoon season. BC concentrations from 1975à2000 relative to 1860à1975 have increased approximately threefold, indicating that BC from anthropogenic sources is being transported to high elevation regions of the Himalaya.
The nature of nitrate at the ice surface
Trace contaminants such as strong acids have been suggested to affect the thickness of the quasi-liquid layer at the ice/air interface, which is at the heart of heterogeneous chemical reactions between snowpacks or cirrus clouds and the surrounding air. We used X-ray photoelectron spectroscopy (XPS) and electron yield near edge X-ray absorption fine structure (NEXAFS) spectroscopy at the Advanced Light Source (ALS) to probe the ice surface in the presence of HNO3 at 230 K.
The mass concentration of volcanic ash from Iceland in European airspace
Data of the Paul Scherrer Institute from the High-Alpine Research Station Jungfraujoch yield important information.The eruption of the volcano Eyjafjallajokull in Iceland has stalled flight traffic in large parts of Europe. Decision makers had to base their decisions mainly on model calculations for the volcanic plume dispersion. How dangerous is this volcanic ash layer for planes?
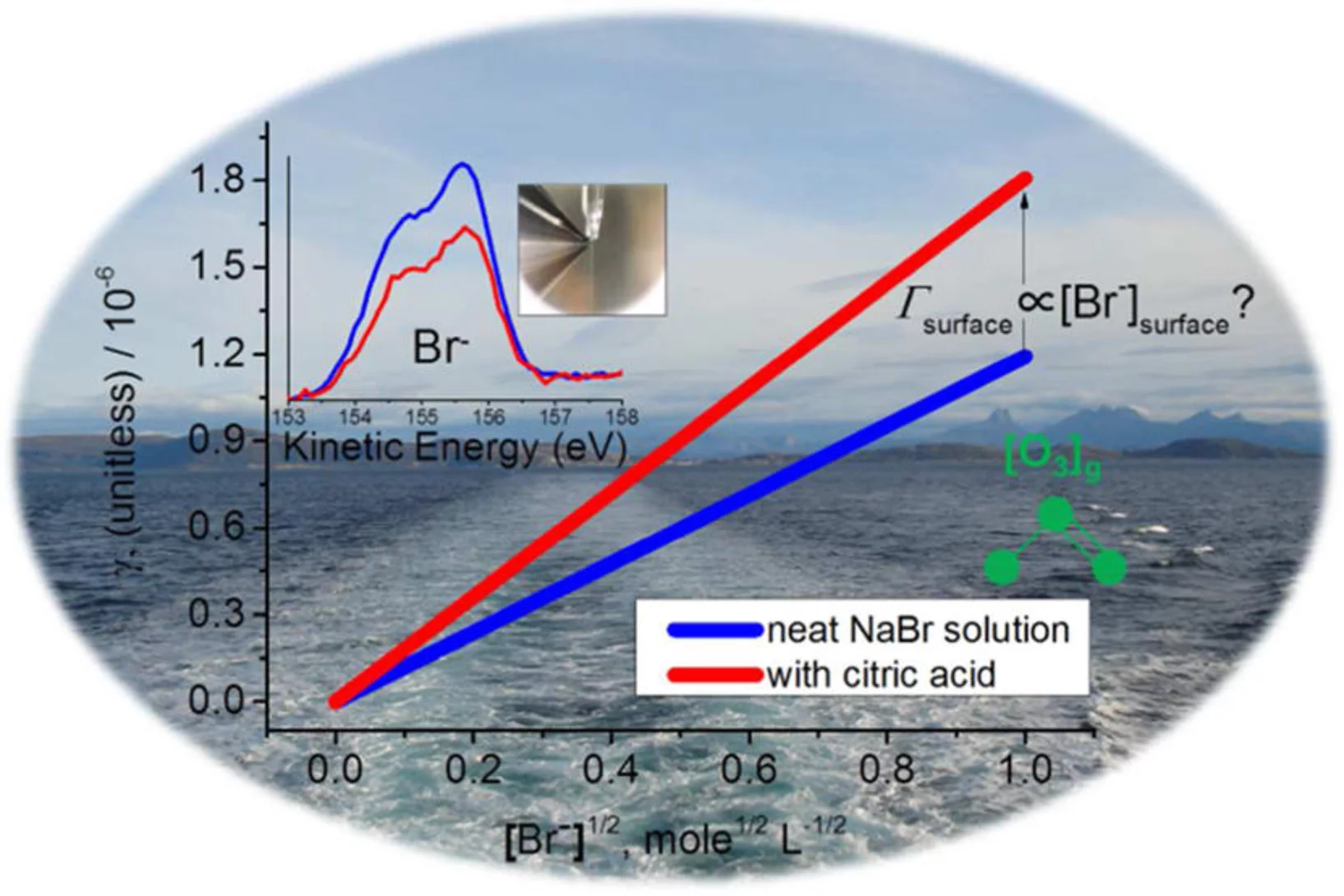
The competition between organics and bromide at the aqueous solution – air interface as seen from ozone uptake kinetics and X-ray photoelectron spectroscopy
A more detailed understanding of the heterogeneous chemistry of halogenated species in the marine boundary layer is required. Here, we studied the reaction of ozone (O3) with NaBr solutions in presence and absence of citric acid (C6H8O7) under ambient conditions. Citric acid is used as a proxy for oxidized organic material present at the ocean surface or in sea spray aerosol.
News from the smog chamber: mechanisms of particle formation in the atmosphere unveiled
Up to the present time, the nucleation or new formation of particles in the atmosphere has been a great enigma. Until recently, research was based on the assumption that sulphuric acid played the central role in particle formation. However, laboratory experiments and field tests have consistently provided conflicting results. In the lab, considerably higher concentrations of sulphuric acid are required for nucleation to take place than in the atmosphere itself. Now scientists from the Paul Scherrer Institute (PSI) have found out the cause for these conflicting results from their smog chamber. These findings will advance climate research to a significant degree.
Mystery solved: how fine particulates are formed in the air
Researchers from the Paul Scherrer Institute, the University of Colorado and 29 other research institutions in various countries have investigated the composition of the organic constituents of the fine particulates found in various regions of the world, and have identified the original substances from which they are formed in each case. For the first time ever, this has enabled them to explain the role played by the individual components of emissions in the development of fine particulates.
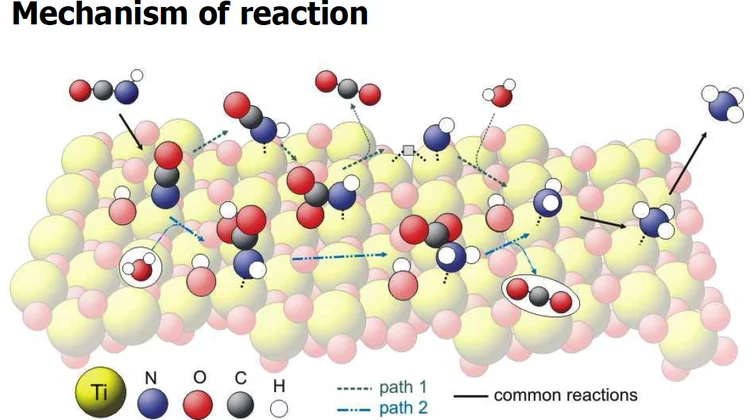
Vibrational Spectra of Adsorbates from DFT
The hydrolysis of isocyanic acid was studied experimentally and theoretically and a reaction mechanism on different catalysts was established. The decreasing NOx emission limits for diesel vehicles impel the further development of the existing NOx deactivation technologies, particularly the selective catalytic reduction (SCR) of nitrogen oxides with urea. In the urea-SCR process, urea is injected into the hot exhaust gas, where it thermally decomposes into isocyanic acid (HNCO) and ammonia.