Laser ablation of polymers was reported first in 1982 by R.Srinivasan et al and Y. Kawamura et al and has been considered as promising method for dry etching of polymers. Very soon the first discussions about the mechanism started, i.e. whether the ablation mechanism is thermal, photothermal or photochemical. Two approaches that can give insights into this complicated processes are single pulse and/or time-resolved methods and the modification or design of polymers for ablation.
Design of Polymers
(with the Laboratory for Functional Polymers, Empa)
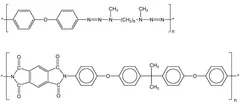
The main goal for designing polymers is the development of photolabile polymers, which are applied as dynamic release layers in laser-induced forward transfer (LIFT) for the deposition of OLED and sensor materials.
Properties of designed polymers:
The main goal for designing polymers is the development of photolabile polymers, which are applied as dynamic release layers in laser-induced forward transfer (LIFT) for the deposition of OLED and sensor materials.
Properties of designed polymers:
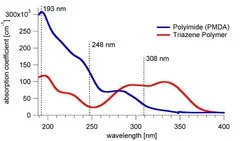
- High absorption coefficient at the irradiation wavelength
- Integration of photochemical active group in the polymer main chain
- Exothermic decomposition
- Large amount of gaseous ablation products, that could act as carrier gas for 'larger' ablation fragments
- 'Low' amount of aromatic groups, which are probably the origin of carbonization
- Introduction of photochemical active groups in the main chain of the polymer
- Decoupeling of the absorption of the active unit from the rest of the polymer
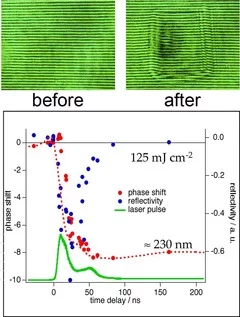
Siemens star produced with 308 nm laser irradiation in TP (left) and PI (right). Sharper features are observed for TP. Also no contamination of the ablated area and the surrounding surface by ablation products is observed for TP compared to PI.
Analysis Methods
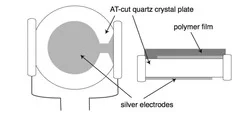
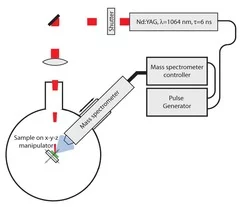
Single-pulse methods
Important results from QCM measurements:
- Carbonisation of surface after the first pulse (change of the ablation rate)
- Desorption of adsorbates at very low fluences
- Decomposition and release of gaseous products from the polymers without structuring
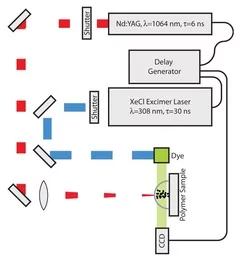
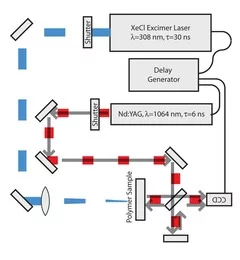
Time-resolved methods
Applications
Polymers as dynamic release layers (DRL) for laser-induced forward transfer (LIFT) of OLED and sensor materials (with the Laboratory for Functional Polymers, Empa)
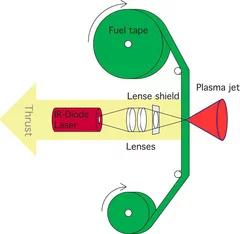
Polymers as fuel for laser based micro-thruster (with Photonic Associates, LLC)
Polymers as fuel for laser based micro-thruster (with Photonic Associates, LLC)
The expansion velocity of selected ablation products was measured with different methods:
- Shockwave expansion velocity measured with Shadowgraphy at low irradiation fluence in ambient conditions
- The kinetic energy of C+-ions with Mass spectrometry
- The expansion velocity of neutral Hydrogen by observing the expansion of the H-Balmer line with Plasma Emission spectrometry
| Polymer | Shadowgraphy [m/s] | Mass Spectrometer [m/s] | Plasma Emission [m/s] |
|---|---|---|---|
| GAP+C | 710 | 28600 | 46000 |
| GAP+IR | 850 | 29300 | 36700 |
| PVN+C | 1080 | 25100 | 31100 |
| PVC+C | 630 | 25900 | 48300 |
- GAP showed the best performance as fuel for the micro-thruster
- Chemically stored energy is released by decomposing polymer
- Efficiency of 50% measured for PVC
- Worst performance observed for PVN (Strong thermal effects were observed)
Surface Modification
Excimer Lamp
Incoherent photon sources, such as mercury or excimer lamps can be used for various applications, e.g. synthetic photochemistry, surface modification and structuring. Mercury lamps are the classical UV-photon source (lines at 365 nm and 254 nm) while excimer lamps are a newer development (1980ies). The latter emit in the visible, UV and even vacuum UV. They do not produce the high photon fluxes of lasers, but are capable of emitting in quasi-CW mode over large areas. We currently apply three different excimer lamps, which are shown below. From left to right: Xe2* at 172 nm, KrCl* at 222 nm, and XeCl* at 308 nm.
Incoherent photon sources, such as mercury or excimer lamps can be used for various applications, e.g. synthetic photochemistry, surface modification and structuring. Mercury lamps are the classical UV-photon source (lines at 365 nm and 254 nm) while excimer lamps are a newer development (1980ies). The latter emit in the visible, UV and even vacuum UV. They do not produce the high photon fluxes of lasers, but are capable of emitting in quasi-CW mode over large areas. We currently apply three different excimer lamps, which are shown below. From left to right: Xe2* at 172 nm, KrCl* at 222 nm, and XeCl* at 308 nm.
Modification of Poly(dimethylsiloxane)
Recently excimer lamps have been used to modify an important technical polymer i.e. poly-dimethylsiloxane (PDMS). Poly(dimethylsiloxane)s (PDMS) are widely used as coatings in a variety of fields including biomedical applications, such as membrane technology, microlithography, optics and dielectrics.
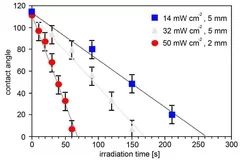
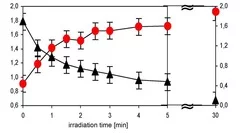
Cross-linked poly(siloxane)s possess unique mechanical, nearly ideal elastomer and optical properties, low weight, high durability, high gas permeability and excellent water repellency. The modification of the hydrophobic PDMS to hydrophilic SiOx opens an additional wide range of applications i.e. in microelectronics and coating technology for medical devices. Transformation of PDMS to SiOx structure have been achieved by irradiation with a Xe2 excimer lamp at 172 nm in air. The modification of the surface properties have been analyzed in detail by contact angle measurements of water, which reveal a fast decrease of the contact angle as a function of irradiation time and intensity . This change of the contact angle is associated with a change of the chemical composition of the surface, which has been measured by XPS. The surface layer becomes enriched in oxygen and depleted in carbon to reach an O/Si ratio of almost 2.
Recently excimer lamps have been used to modify an important technical polymer i.e. poly-dimethylsiloxane (PDMS). Poly(dimethylsiloxane)s (PDMS) are widely used as coatings in a variety of fields including biomedical applications, such as membrane technology, microlithography, optics and dielectrics.
Cross-linked poly(siloxane)s possess unique mechanical, nearly ideal elastomer and optical properties, low weight, high durability, high gas permeability and excellent water repellency. The modification of the hydrophobic PDMS to hydrophilic SiOx opens an additional wide range of applications i.e. in microelectronics and coating technology for medical devices. Transformation of PDMS to SiOx structure have been achieved by irradiation with a Xe2 excimer lamp at 172 nm in air. The modification of the surface properties have been analyzed in detail by contact angle measurements of water, which reveal a fast decrease of the contact angle as a function of irradiation time and intensity . This change of the contact angle is associated with a change of the chemical composition of the surface, which has been measured by XPS. The surface layer becomes enriched in oxygen and depleted in carbon to reach an O/Si ratio of almost 2.