The conversion efficiency for green hydrogen production in a polymer electrolyte water electrolyzer (PEWE) is strongly influenced by the ohmic cell resistance and therefore the thickness of the membrane. The use of thin membranes (~50 micron or below) is limited by gas crossover of H2 and O2, which can lead to the formation of an explosive gas mixture. The incorporation of a Pt recombination catalyst provides remedy and allows a more dynamic operating mode (cf. Highlight 03/2022). However, the presence of Pt nanoparticles leads to an increase in the rate of membrane degradation. Therefore, we have additionally doped the membrane with cerium-zirconium-oxide (CZO) nanoparticles, which act as radical scavenger. The rate of membrane degradation can thus be reduced.
The use of thin membranes with a thickness of 50 mm or below leads to a reduction of the ohmic loss of the polymer electrolyte water electrolyzer vs. membranes with a more traditional thickness between 150 and 200 micron. The produced H2 and O2, however, passes more easily through a thin membrane, which can lead to the formation of an explosive gas mixture. The situation is particularly critical on the anode side of the cell, where O2 is electrochemically produced, since the diffusivity of H2 through the membrane is higher than that of O2 and the rate of O2 production is only half of that of H2. The flammability limit is 4% H2 in O2, and a safety limit of 2% H2 in O2 is typically used. To mitigate the formation of an explosive gas mixture, a gas recombination catalyst, in the form of Pt nanoparticles, can be incorporated into the membrane. Crossover H2 and O2 will react to H2O on its surface (hence ‘recombination catalyst’), thus the amount of gas reaching the opposite side is reduced.
During the reaction of H2 and O2 on the Pt surface OH-radicals are formed as intermediates, which have a high oxidative strength and attack the membrane polymer, leading to chain breakdown, which will eventually trigger membrane failure. Commercial perfluorinated membranes used in fuel cells contain radical scavengers, such as Ce(III) ions or cerium oxide (‘ceria’) nanoparticles, which quench the radicals according to the reaction ·OH + Ce(III) + H+ H2O + Ce(IV), thus protecting the ionomer against radical attack. Ceria is preferred over cerium-ions as radical scavenger, because i) it does not displace protons from the membrane, and ii) it does not migrate under the influence of the electric field, unlike cerium-ions. However, ceria (CeO2-d) is thermodynamically unstable at low pH and the potentials prevalent in an electrolyzer. Therefore, in the presented approach, we used a mixed oxide, cerium-zirconium oxide (CZO), as a membrane dopant and radical scavenger.
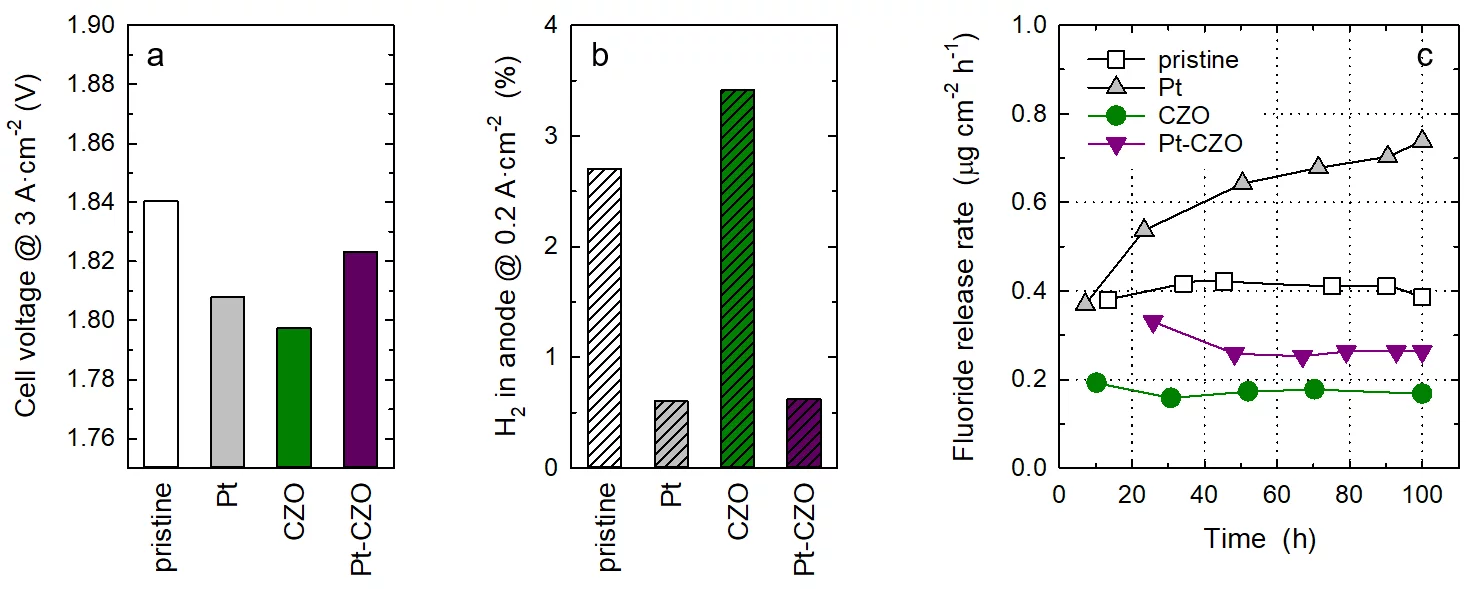
Membranes were prepared by solution casting of Nafion™ dispersion onto a glass plate, followed by drying and curing, to obtain a membrane thickness of around 60 micron. In addition to a pristine, undoped membrane, a membrane containing Pt-black nanoparticles as recombination catalyst with a loading of 0.04 mgPt×cm−2 was prepared. Another series of membranes were prepared that contained commercial CZO nanoparticles as radical scavenger up to a content of 10 wt-%. At a dopant level of 5 wt-% and above, an impact of cell performance was observed, therefore subsequent experiments were performed with a CZO content of 1 wt-%. Finally, a membrane co-doped with Pt at a loading of 0.04 mgPt×cm−2 and 1 wt-% CZO was prepared. The membranes were coated with Pt-based cathode catalyst layer and a IrTiOx-based anode catalyst layer to obtain catalyst coated membranes (CCMs) with an active area of 25 cm2. CCMs were assembled into single cells, using a sintered titanium porous transport layer (PTL) on the anode and a carbon based gas diffusion electrode (GDL) on the cathode. Cell tests were performed at a temperature of 80°C. Polarization curves were recorded after conditioning, from which the cell voltage values at a current density of 3 A×cm−2 were extracted (see Figure, Panel a). The doped membranes did not have an adverse effect on cell performance. However, the gas crossover was significantly reduced in the presence of the Pt recombination catalyst (Panel b). In extended cell operation over 100 h at a constant current density of 2 A×cm−2, the rate of membrane degradation was assessed by measuring the fluoride release rate (FRR), as radical attack of Nafion™ leads to the emission of fluoride ions. As explained above, the incorporation of the Pt recombination catalyst leads to an enhanced rate of membrane degradation, which moreover appears to increase over time. Doping of membranes with CZO reduces membrane degradation through radical scavenging. Although the FRR of the Pt-CZO co-doped membrane is higher than that of the CZO-only doped membrane, the values are still lower than those of the pristine Nafion™ membrane. This indicates that co-doping of membranes with a recombination catalyst and a radical scavenger improves membrane functionality for water electrolyzer applications, highlighting potential design strategies for next-generation membranes.
Contact
PD Dr. Lorenz Gubler
Head Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publications
Platinum and Cerium-Zirconium Oxide Co-Doped Membrane for Mitigated H2 Crossover and Ionomer Degradation in PEWE
Zheyu Zhang, Zongyi Han, Andrea Testino, Lorenz Gubler
J. Electrochem. Soc. 169, 104501 (2022).
DOI: 10.1149/1945-7111/ac94a3
Additionally, please view the PSI Electrochemistry Scientific Highlight published in March 2022:
https://www.psi.ch/en/lec/scientific-highlights/enabling-the-use-of-thi…
Acknowledgement
Swiss Federal Office of Energy (grant number SI/502174).