The materials class of ionic liquids has attracted an enormous research interest in the past few years. This huge interest in these liquids is mainly due to the fact that ionic liquids offer very often an attractive alternative to conventional reaction media. The main tool for investigating the dynamics occurring in these liquids is quasi-elastic (QENS) and inelastic neutron scattering (INS). QENS allows for studying localized dynamical processes (rotations, librations, jump-processes etc.), while INS provides information on interionic and magnetic interactions. All processes depend on the geometry of the ions and the local environment given by surrounding neighbors. In order to fully benefit from neutron scattering techniques we use deuteration (in collaboration with chemists from Saarland University in Germany) to highlight or mask parts of the molecules (or complete molecules) under investigation. In collaboration with colleagues from University College Dublin, we use computer simulation techniques (MD simulations, DFT calculations) to better understand the dynamical processes and interactions in IL systems.
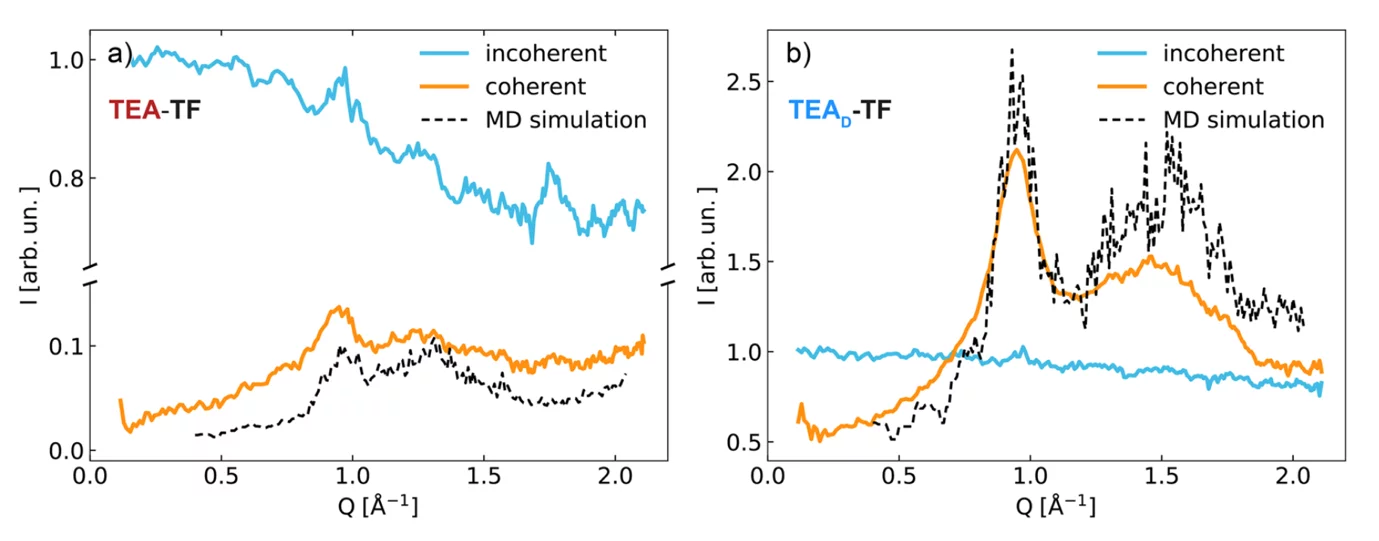
Fig. 1 shows results from neutron scattering and simulations on the IL system triethylammonium (TEA) trifluoromethanesulfonate (TF). Using polarized neutrons, we separated coherent and incoherent scattering contributions and the structural information contained in the coherent part is compared to corresponding MD results.
Like several other families of organic compounds, ionic liquids (ILs) containing appropriate ions, may display long-range magnetic ordering in the crystal phase, arising below a Curie or Néel temperature in the range from a few to about 50 K. This is especially true of compounds containing metal-organic groups, but magnetism occurs also in a few systems having extended π-bonding. While generally fairly low, the Curie or Néel temperature shows large relative variations upon changing the anion. It is known, for instance, that in imidazolium-based magnetic ionic liquids (MILs), replacing Cl– by Br– in the [FeCl4]– anion increases the Néel temperature by a factor between 1.38 to 3.10, depending on the involved cation. Among those imidazolium–based MILs with the tetrahaloferrate(III) anion that display long-range magnetic ordering, the magnetic interaction responsible for these features acts via direct or indirect super-exchange path-ways, along Fe–X⋯X–Fe (X=Br/Cl) bridges, involving the halides and in some cases the imidazolium ring in the cation.
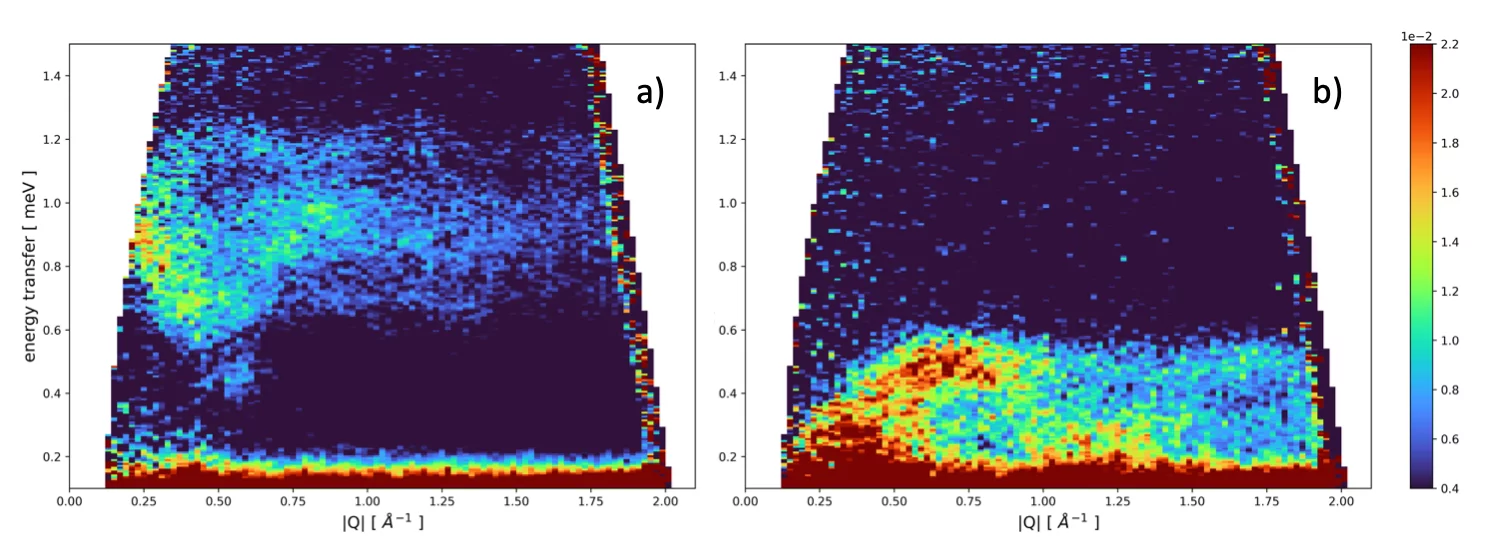
One of the questions we aim to answer is how the magnetic interaction influences the structure of the MILs in the liquid phase. We started our research with imidazolium–based MILs with tetrahaloferrate(III) anions and will later extend our investigations to phosphonium- and/or ammonium–based MILs with both tetrahaloferrate(III) and rare–earth containing anions. The latter MIL families are far less investigated with respect to magnetic, structural and dynamic properties of the compounds both in the liquid and crystalline state. As an example of the low-temperature behavior of MILs we show in Fig. 2a) the scattered intensity of the system 1-ethyl-3-methylimidazolium tetrachloroferrate(III) measured at 1.5 K after cooling the system from the liquid state to 1.5 K in the presence of an 8T vertical magnetic field . The dispersion of low-lying magnetic excitations between 0.6 and 1.3 meV are clearly visible. Switching off the applied magnetic field at 1.5 K, the shape and position in energy of the observed excitations change considerably (see Fig. 2b). The understanding of the exact origin of the observed excitations and how the composition of the MILs under investigation, i.e. the specific interactions will influence the excitations is purpose of current investigations
References
- Busch, M., Hofmann, T., Frick, B., Embs, J.P., Dyatkin, B., Huber, P., "Ionic liquid dynamics in nanoporous carbon: A pore-size- and temperature-dependent neutron spectroscopy study on supercapacitor materials", Physical Review Materials, 4 (2020) 055401
- Mora Cardozo, J.F., Embs, J.P., Benedetto, A., Ballone, P., "Equilibrium Structure, Hydrogen Bonding, and Proton Conductivity in Half-Neutralized Diamine Ionic Liquids", Journal of Physical Chemistry B, 123 (2019) 5608
- Burankova, T., Mora Cardozo, J.F., Rauber, D., Wildes, A., Embs, J.P., "Linking Structure to Dynamics in Protic Ionic Liquids: A Neutron Scattering Study of Correlated and Single-Particle Motions", Scientific Reports, 8 (2018) 16400
- Mora Cardozo, J.F., Burankova, T., Embs, J.P., Benedetto, A., Ballone, P., "Density Functional Computations and Molecular Dynamics Simulations of the Triethylammonium Triflate Protic Ionic Liquid", Journal of Physical Chemistry B, 121 (2017) 11410
- Burankova, T., Simeoni, G., Hempelmann, R., Cardozo, J.F.M., Embs, J.P., "Dynamic Heterogeneity and Flexibility of the Alkyl Chain in Pyridinium-Based Ionic Liquids", Journal of Physical Chemistry B, 121 (2016) 240
- Burankova, T., Hempelmann, R., Fossog, V., Ollivier, J., Seydel, T., Embs, J.P., "Proton Diffusivity in the Protic Ionic Liquid Triethylammonium Triflate Probed by Quasielastic Neutron Scattering", Journal of Physical Chemistry B, 119 (2015) 10643
- Burankova, T., Hempelmann, R., Wildes, A., Embs, J.P., "Collective ion diffusion and localized single particle dynamics in pyridinium-based ionic liquids", Journal of Physical Chemistry B, 118 (2014) 14452
Collaboration
- Prof. Rolf Hempelmann, Saarland University (UdS), Germany
- Dr. Daniel Rauber, Saarland University (UdS), Germany
- Prof. Dr. Pietro Ballone, University College Dublin (UCD), Ireland
- Dr. Antonio Benedetto, UCD, Ireland
Funding
Associated junior researchers
- Dr. Tatsiana Burankova, PhD Saarland University, 2015
- Dr. Juan F. Mora Cardozo, PhD University of Geneva, 2019