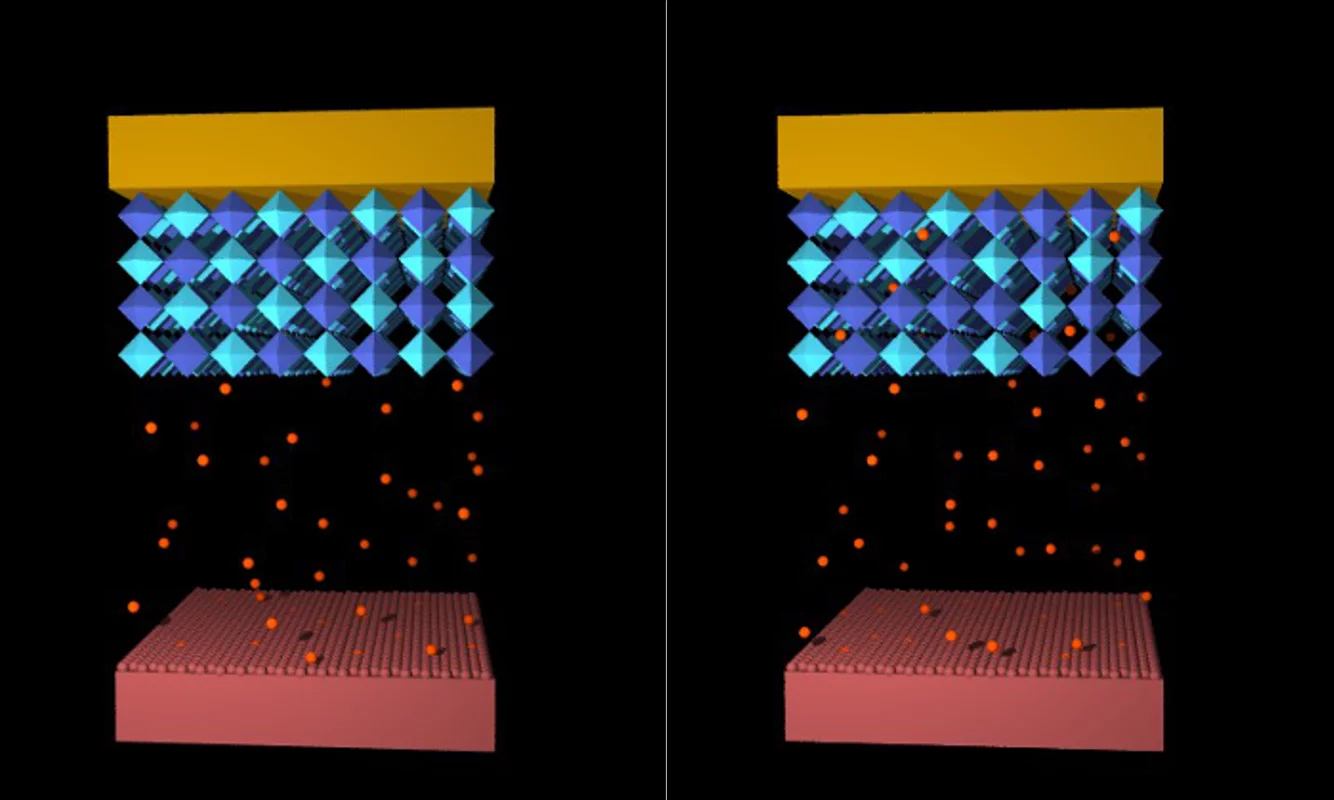
The basic idea of this project is to electrochemically (slightly) change the composition of a material, which is close to a compositional magnetic phase transition. In the ideal case, this way one can reversibly switch between states with and without net magnetisation. The total set-up and operation scheme resembles a battery, where one electrode is the material of interest.
Our model system is LaxSryMnO3 because of its room temperature FM/PM transition for x = y = 0.5. The essential mechanism for this transition is the ordering of Mn3+ and Mn4+ ions at. Doping the system with Li shifts the ratio of this two ions and thus the condition for ordering. Figure 1 shows this concept in an artistic way.
We succeeded to synthesize thin films of LaxSryLizMnO3, either with z=0, x+y=1 on the PM side of the transition, or with x+y+z=1 on the FM side. These were used as electrodes battery-like set ups, both with liquid and solid electrolytes. Magnetometry and x-ray absorption spectroscopy both showed a partial reversibility of the lithiation state, and polarised neutron reflectometry revealed a change in the mean magnetic moment of Mn of up to 1 μB. It is not clear jet, if this is associated with complete switching.
Further neutron reflectometry (NR) studies are planned. NR is ideally suited for this problem since it can probe the net magnetisation and the compositional changes with a high spatial resolution during operation.
Publications
-
Bimashofer G, Smetaczek S, Gilardi E, Schneider CW, Limbeck A, Lippert T, et al.
Growth of LixLaySrzMnO3 thin films by pulsed laser deposition: complex relation between thin film composition and deposition parameters
Applied Physics A: Materials Science and Processing. 2021; 127(6): 473 (8 pp.). https://doi.org/10.1007/s00339-021-04506-9
DORA PSI
Collaboration
- Th. Lippert, Ch. Schneider, Ch. Klauser, NUM/PSI (sample preparation)
- C. Vaz, A. Clark, SLS/PSI (x-ray measurements)
- S. Smetaczek, A. Limbeck, TU Wien, Austria (sample characterization)
Funding
- SNSF Project No. 200021_169704
Associated junior researchers
- Gesara Bimashofer, PhD ETH Zürich, 2021