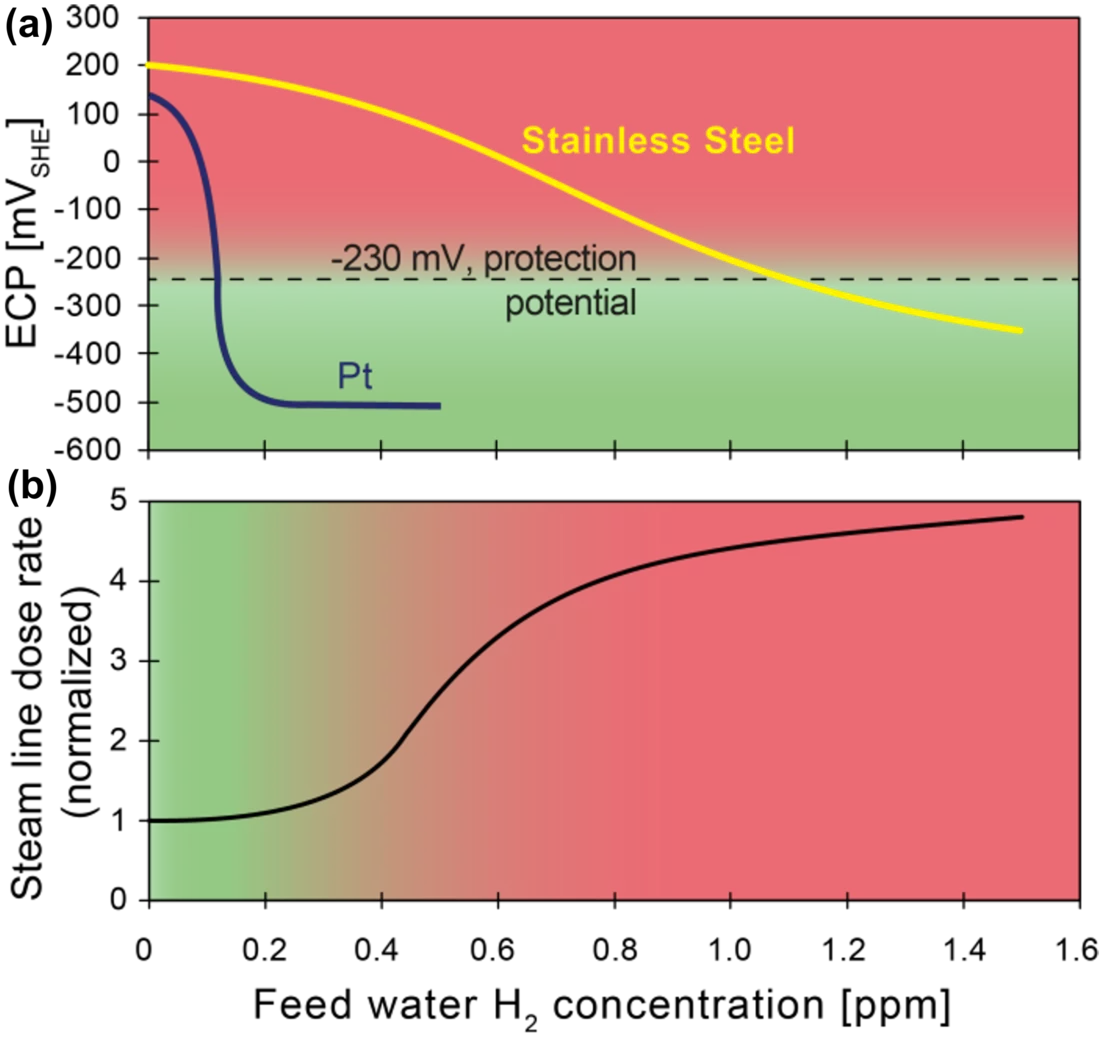
The formation and growth of cracks by stress corrosion cracking (SCC) in reactor internals and recirculation pipes due to the highly oxidising environment (O2 and H2O2, formed by radiolysis of water) is a serious issue in boiling water reactors (BWRs). At first, SCC mitigation was attempted by injecting H2 into the feed water, a method termed hydrogen water chemistry (HWC), where the injected H2 recombines with the H2O2 and O2 to water and reduces the electrochemical corrosion potential (ECP), and consequently the SCC susceptibility. Several disadvantages of the injection of high amounts of H2 (e.g., increase of the main steam line dose rates due to release of volatile N16 carried over to the turbine), have led to the development of noble metal (platinum, Pt) additions to the reactor feed water. With injection of a much smaller amount of H2, the noble metal particles of a few nanometres in size, formed in-situ, work as catalysts for the efficient reduction of the oxidizing species formed by radiolysis, and thus lower the ECP and SCC susceptibility, without the negative side-effects of classical HWC (Figure 1). This SCC mitigation requires a sufficient surface coverage with homogeneously distributed very small Pt particles at the critical locations. Even though the Online Noble Metal Chemical (OLNC) application technology is in use in both Swiss BWR plants, as well as in many other BWRs worldwide [1], until recently, very little was known about the Pt deposition and (re‑)distribution behaviour in BWRs. Reliable information on the local Pt coverage of the plant surfaces is crucial, both to evaluate the effectiveness of the method and to control the application process (intervals, amount of Pt, etc.).
In this context, a joint effort between PSI, the Swiss Federal Nuclear Safety Inspectorate (ENSI) and the nuclear power plants of Leibstadt (KKL) and Mühleberg (KKM) in Switzerland was initiated. This project, called NORA (started in 2010), is aimed at obtaining new phenomenological insights and a better basic understanding of the Pt distribution and deposition behaviour in BWRs as well as providing vendor independent insights and ways to optimise the process. It includes also the development of a non-destructive technique to assess the Pt coverage on (plant) surfaces. Beside the work in the laboratory at PSI, experiments are also performed at KKL to collect data from full-scale applications.
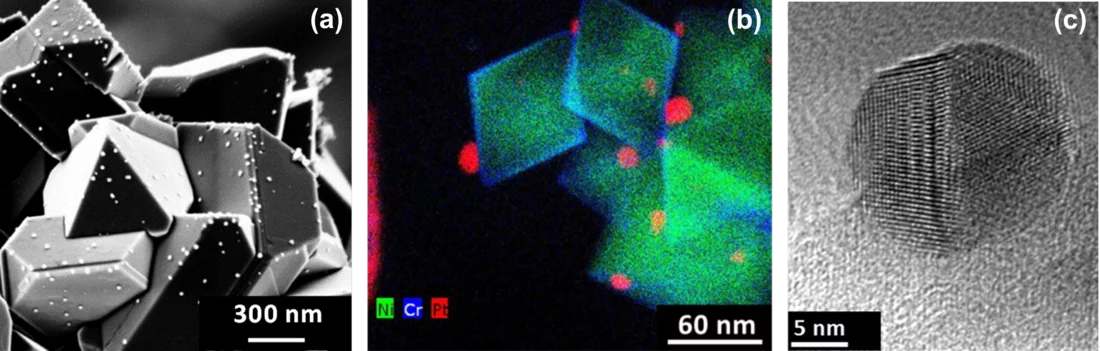
To be independent of the technical, economic and time constraints that are unavoidable when doing experiments in a plant, but still obtain data relevant to plant operation it is essential to have an adequate lab setup. The Laboratory for Nuclear Materials at PSI has assembled a sophisticated high-temperature water loop facility to reproduce the water chemistry of a BWR including OLNC applications. After a Pt application experiment which involves Pt injection (as Na2Pt(OH)6) into the hot water at concentrations down to some 100 ppt only, the size, shape and distribution of Pt nanoparticles, deposited on the stainless steel specimens, are characterised by using high-resolution Scanning Electron (SEM) and Transmission Electron (TEM) Microscopy techniques (Figure 2). Quantitative values for the Pt surface loading are obtained by Laser Ablation - Inductively Coupled Plasma – Mass Spectrometry (LA-ICP-MS).
In the following paragraph some selected results are briefly outlined:
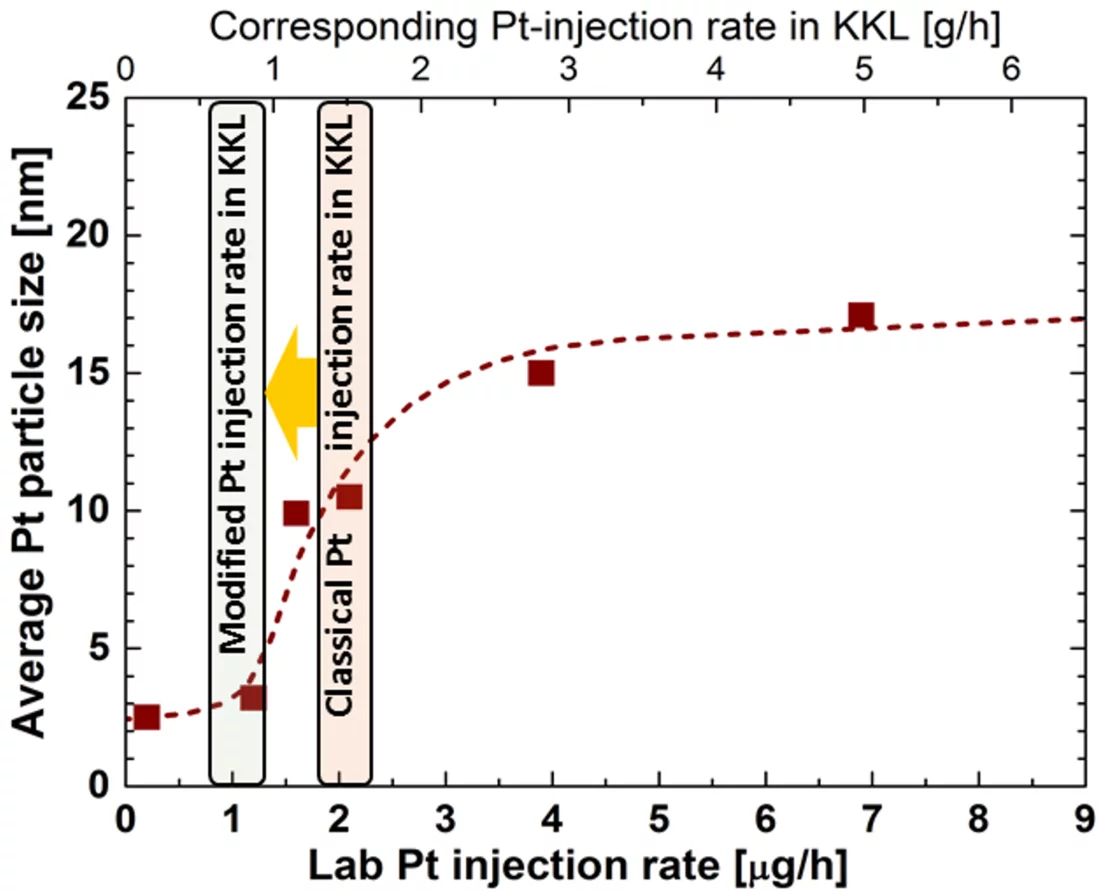
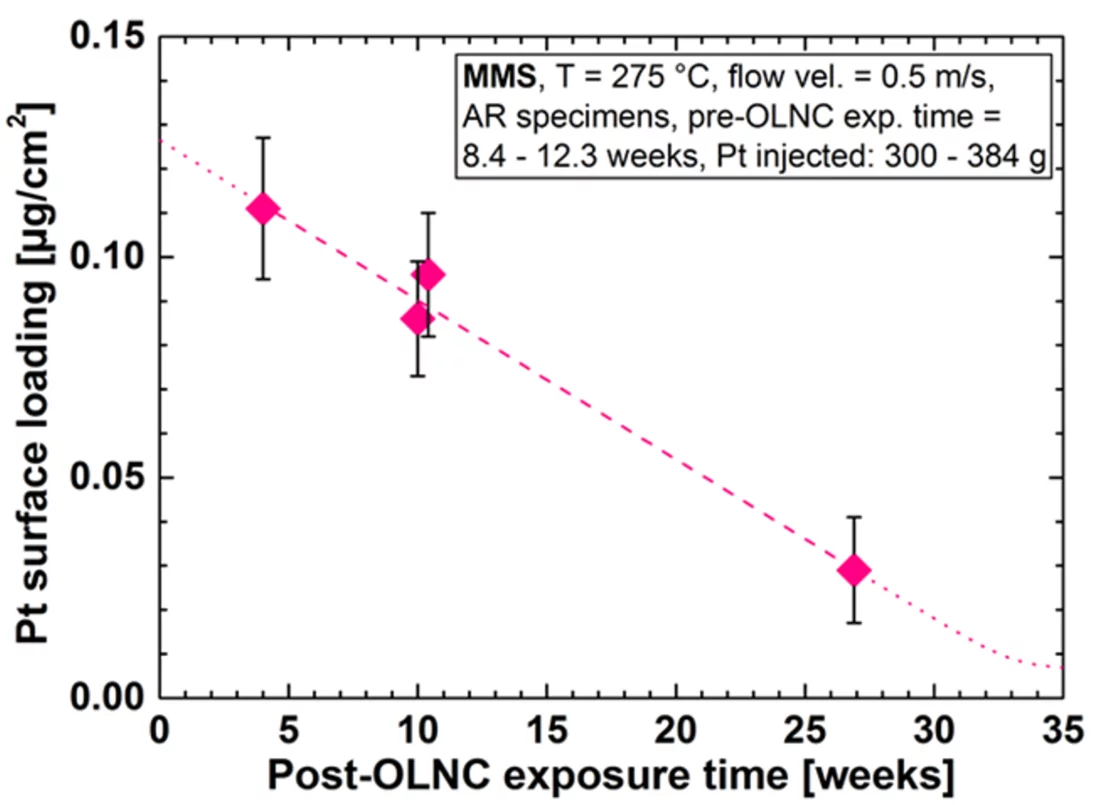
One outcome of the NORA project is that the Pt nanoparticles are pure metal and stick to the oxide layer on the stainless steel regardless of the oxide composition (Figure 2b). It could also be shown that the size of the Pt particles decreases with decreasing injection rate of the Pt into the feed water (Figure 3), whereas smaller particles are able to deposit more evenly on the component surfaces and thus are advantageous for this SCC mitigation technology. Exposure of coupon specimens to the reactor water of KKL revealed that the Pt, once deposited on the steel surfaces by OLNC application, slowly erodes away with time (Figure 4). OLNC re-applications are needed to maintain a good protection level against SCC. Results from lab investigations have confirmed this. Since both, the Pt deposition and Pt erosion behaviour are dependent on the flow conditions of the reactor water (which strongly vary in a reactor), they were also investigated in detail. For this purpose, experiments with rotating disks, covering a wide range of flow conditions, from laminar to turbulent on a single specimen under identical water chemistry, were performed. The Pt surface loading values at different positions on one disk is shown exemplarily in Figure 5. Low loading values were measured at the centre where slow laminar flow is present, whereas the Pt loading increases towards the edge of the disk with highly turbulent flow. Beside this, many other parameters, as e.g., possible negative side effects of the OLNC technology on the fuel rod cladding behaviour (corrosion and H2 uptake) are studied [2-5].
Based on results from the NORA project, plant recommendation could already be given, which were directly applied in the Swiss BWR plants: OLNC application is best performed with low Pt injection rates under reducing water chemistry conditions and be repeated on a regular basis during a plant cycle to compensate for loss of Pt from the surfaces.
Contact
Stefan RitterLaboratories for Nuclear Materials (LNM)
Paul Scherrer Institut
E-mail: stefan.ritter@psi.ch
Original Publications
[1] P.V. Grundler and S. Ritter, "Noble Metal Chemical Addition to BWRs for Stress Corrosion Cracking Mitigation: Theoretical Insights and Applications", PowerPlant Chemistry, 2014, 16(2), pp. 76-93.[2] S. Ritter, P.V. Grundler, L. Veleva, G. Ledergerber, and R. Pathania, "Assessment of the Platinum Deposition Behaviour on Stainless Steel Surfaces in a Boiling Water Reactor Plant", Corrosion Engineering Science and Technology, 2017, accepted for publication.
[3] H.F. Gu, B. Niceno, P.V. Grundler, M. Sharabi, L. Veleva, and S. Ritter, "Computational Study of Platinum Nanoparticle Deposition on the Surfaces of Crevices", Nuclear Engineering and Design, 2016, 304, pp. 84-99.
[4] P.V. Grundler, L. Veleva, A. Ramar, and S. Ritter, "Effect of Flow Conditions on the Deposition of Platinum Nanoparticles on Stainless Steel Surfaces", Corrosion, 2015, 71(1), pp. 101-113.
[5] P.V. Grundler, L. Veleva, and S. Ritter, "Pt: Key to Improved SCC Mitigation", Nuclear Engineering International, 2014, 59(725), pp. 33-35.