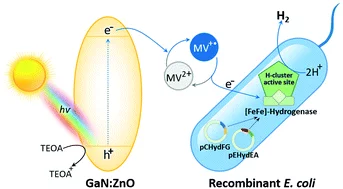
The need for sustainable, renewable and low-cost approaches is a driving force behind the development of solar-to-H2 conversion technologies. This study aims to develop a new strategy using a visible-light photocatalyst coupled to a biocatalyst for H2 production. Photocatalytic methyl viologen (MV2+) reduction activity was investigated to discover active oxynitrides. In comparative studies with LaTiO2N, BaTaO2N and Ta3N5, it was revealed that the suitable surface area, band gap and band edge potentials are some physical factors that are responsible for the photocatalytic behaviors of GaN:ZnO in MV2+ reduction. The activity is enhanced at higher concentrations and the alkaline pH of triethanolamine (TEOA). The expression of an active [FeFe]-hydrogenase from Escherichia coli (Hyd+E. coli) as a recombinant biocatalyst was confirmed by its MV˙+-dependent H2 production activity. In the photobiocatalytic system of GaN:ZnO and Hyd+E. coli, the rate of H2 production reached the maximum level in the presence of MV2+ as an electron mediator at neutral pH as a biocompatible condition. The present work reveals a novel hybrid system for H2 production using visible-light active GaN:ZnO coupled to Hyd+E. coli, which shows the feasibility of being developed for photobiocatalytic H2 evolution under solar light.
Facility: LMX, WPI-I2CNER
Reference: N. Kosem et al., Catal. Sci. Technol. 10, 4042-4052 (2020)
Read full article: here