The long-lived nuclide 79Se plays a key role in safety assessments for underground radioactive waste repositories. As oxidized anion (SeIVO32- or SeVIO42-) selenium is highly soluble and consequently mobile in a repository environment. On the other hand, it is sparingly soluble and thus less mobile when it occurs in reduced forms such as metallic Se0 or selenide (Se-II).
Due to the low fission yield, the chemical behaviour of Se isotopes during nuclear power plant operation is poorly known. In general, Se is assumed to diffuse out of the fuel grains due to the high thermal gradient and to migrate toward the periphery of fuel pellets, similarly to volatile elements such I and Cs. As a consequence a significant part of the 79Se inventory in spent fuel would be readily accessible to aqueous leaching in the radioactive waste repository environment. However, contrary to expectations, leach experiments conducted by PSI's Hot Laboratory (AHL) failed to show measurable Se release after exposing spent UO2 fuel samples to aqueous solution for up to one year.
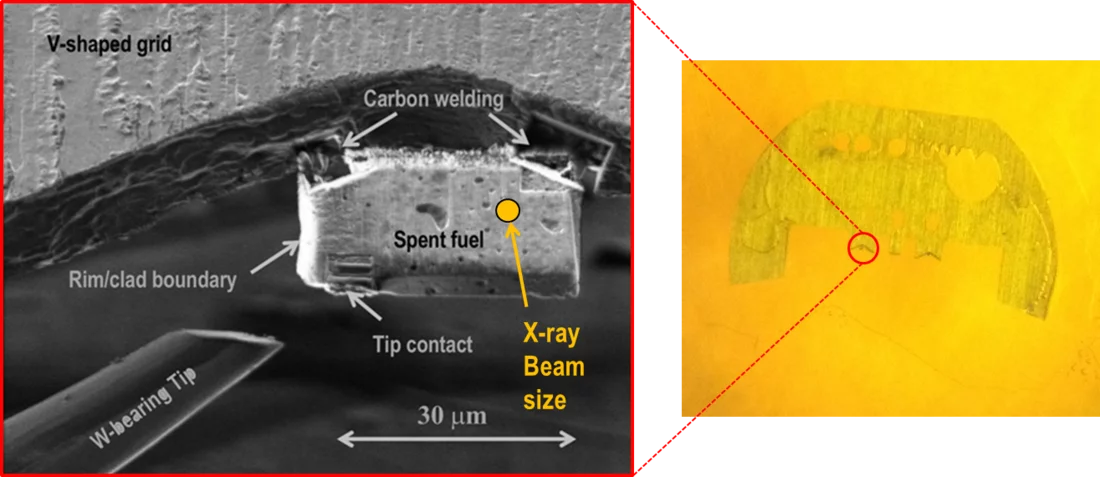
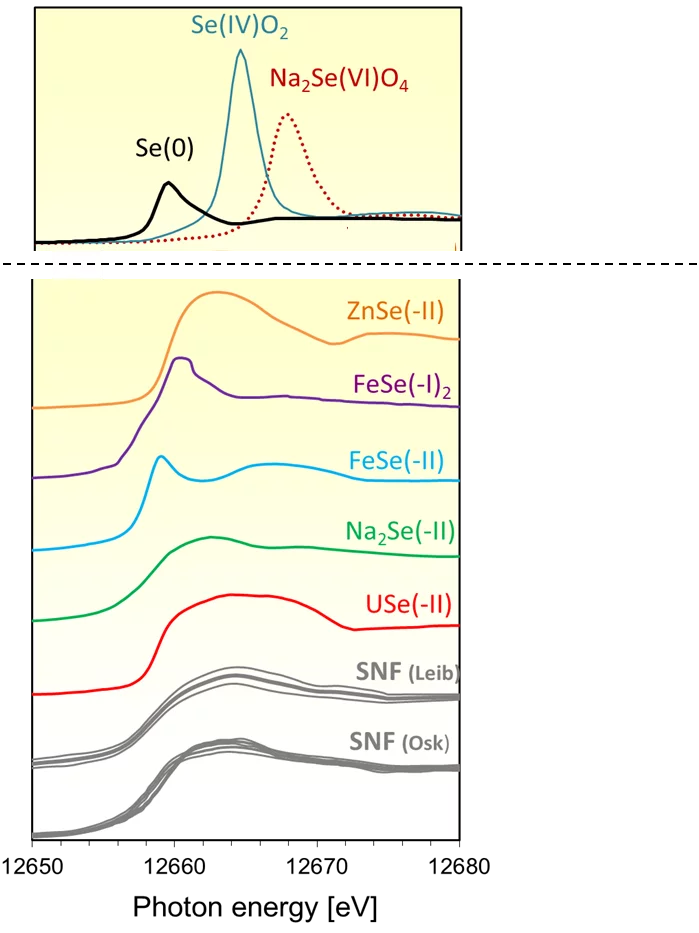
In order to explain this result, X-ray absorption measurements (XANES) were carried out at the microXAS beamline at SLS on specially prepared micro samples of UO2 spent fuel (Figure 1). The comparison of the spent fuel spectra with those of Se reference compounds (Figure 2) suggests that selenium occurs in the spent fuel as sparingly soluble, almost immobile selenide (Se-II) ion, with a coordination environment similar to that in USe. Moreover, the occurrence of soluble, oxidized Se can be ruled out.
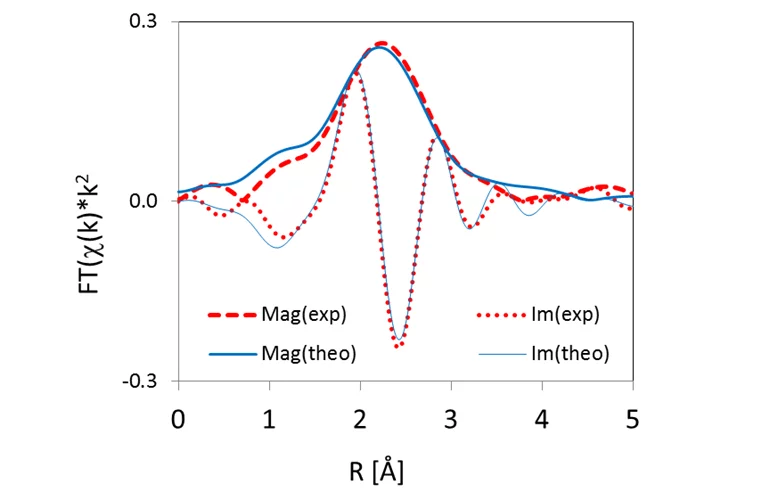
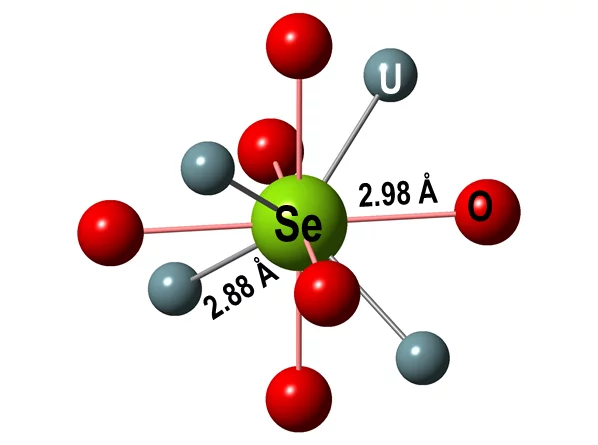
This empirical finding was consolidated by theoretical XANES spectra, calculated for atomic clusters with a central Se absorber occupying specific lattice sites in the UO2 crystal structure. The clusters were either computed ab initio using quantum mechanical methods at LRS or obtained via geometrical optimization of the experimental spectra (Figure 3). The results are consistent with Se replacing vacancies or oxygen in the UO2 structure (Figure 4), leading to Se coordination environments similar to those of U selenides.
In the light of these results one can conclude that the release of 79Se in a geological repository will be controlled by the very slow dissolution rate of the spent fuel matrix and by the low solubility of selenide. These results provide not only an explanation of the failure to detect selenium in the leach experiments, they are also essential to reliably define the release rate of 79Se from spent fuel by eliminating unnecessary conservatism in the source term parameters of the safety case.
Additional information
Environmental Science: Processes & Impacts blog, 27 October 2015
Contact
Dr. Enzo Curti
Transport Mechanisms Group
Laboratory for Waste Management (LES)
Paul Scherrer Institut
Telephone: +41 56 310 2416
E-mail: enzo.curti@psi.ch
Original Publications
Characterization of selenium in UO2 spent nuclear fuel by micro X-ray absorption spectroscopy and its thermodynamic stability
E. Curti, A. Puranen, D. Grolimund, D. Jädernas, D. Sheptyakov and A. Mesbah
Environ. Sci.: Processes Impacts 8 September 2015: Vol. 17, pp. 1760–1768
DOI: 10.1039/C5EM00275C
Selenium redox speciation and coordination in high-burnup UO2 fuel: Consequences for the release of 79Se in a deep underground repository
E. Curti, A. Froideval-Zumbiehl, I. Günther-Leopold, M. Martin, A. Bullemer, H.P. Linder, C. N. Borca and D. Grolimund
J. Nucl. Mater. 10 July 2014: Vol. 453, pp. 98–106
DOI: 10.1016/j.jnucmat.2014.07.003