The PSI Laboratory for Electrochemistry studies almost all aspects of electrochemical energy conversion to advance its scientific and technological understanding for sustainable energy systems.
Vision
We aim to be a global leader in advancing electrochemical energy conversion, driving innovation for a sustainable, clean energy future through excellence in research, collaboration, and education.
Mission
We advance electrochemical science and technology by driving innovation in sustainable energy conversion systems and developing advanced materials. By bridging the gap between fundamental research and practical, real-world applications, we ensure that our discoveries lead to tangible impacts. We foster strong, collaborative partnerships across disciplines and industries, leveraging collective expertise to accelerate progress. Additionally, we are committed to empowering the next generation of leaders through comprehensive education and training programs. Ultimately, our efforts are directed towards creating effective, scalable solutions that address the pressing global challenges of energy sustainability.
Strategy
Our approach is centered on research excellence, utilizing state-of-the-art facilities and groundbreaking methods to drive fundamental discoveries and advancements in electrochemical energy technologies. We emphasize collaborative synergy, fostering strong partnerships across academia, industry, and laboratory groups to facilitate knowledge transfer and accelerate innovation. Our focus is on developing next-generation materials and solutions that meet critical challenges in sustainable energy conversion, ensuring enhanced durability and performance. We are equally committed to education and impact, equipping future leaders through comprehensive training programs, active community engagement, and a strong presence in the scientific community. Together, these pillars reflect our unwavering commitment to sustainability, innovation, and leadership in electrochemistry.
Career and Education within the Laboratory for Electrochemistry
Lab News & Scientific Highlights
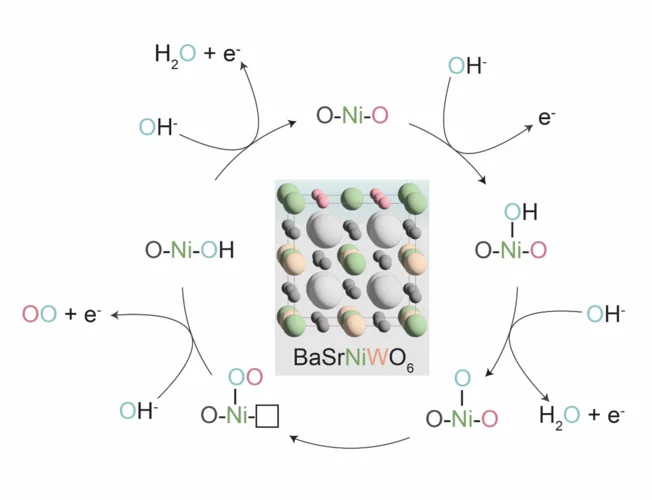
Confining surface oxygen redox in double perovskites for enhanced oxygen evolution reaction activity
Nickel-based double perovskites AA’BB’O6 are an underexplored class of oxygen evolution reaction (OER) catalysts. In particular, BaSrNiWO6 exhibits high oxygen evolution activity, attributed to the evolution of a highly OER active surface phase. The redox transformation of Ni2+(3d8) to Ni3+(3d7) combined with partial W dissolution into the electrolyte drives an in-situ reconstruction of the surface to an amorphized, NiO-like layer, promoting oxygen redox in the OER mechanism.
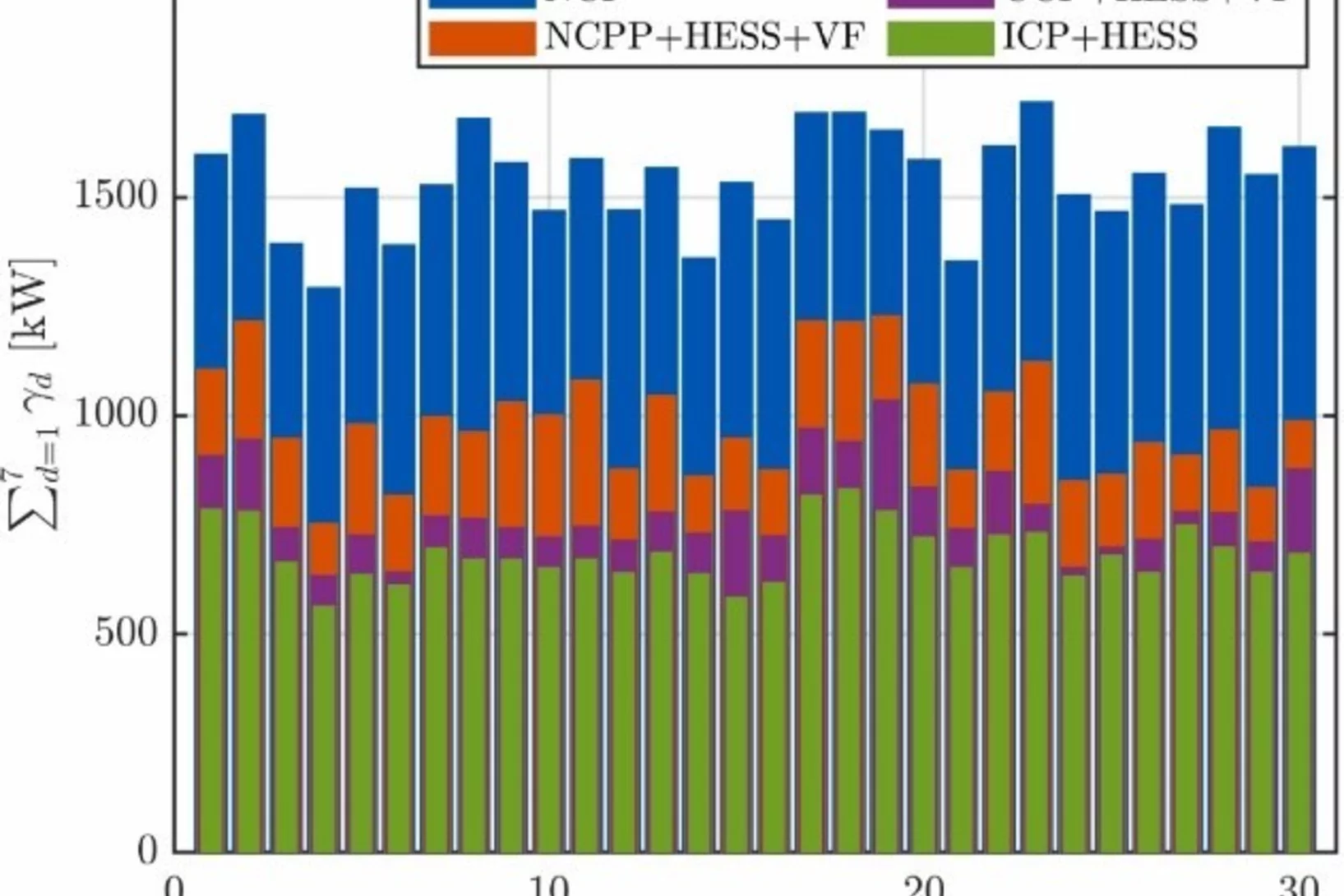
Decentralized hydrogen-based stationary energy storage systems complemented by smart control can provide increased operational flexibility in the energy system
While the electrification of the energy system implies a reduction of greenhouse gas emissions greatly beneficial to society, it can also pose technical challenges. The most notable among these are that the capacity of the local electric grid may be exceeded, along with the occurrence of imbalances between decentralized renewable energy production and final consumption. Hydrogen-based energy storage systems (HESS) are regarded as promising solutions to address these challenges. However, the feasibility has not been demonstrated and the involved processes are not well characterized on a technical relevant power level, so far.
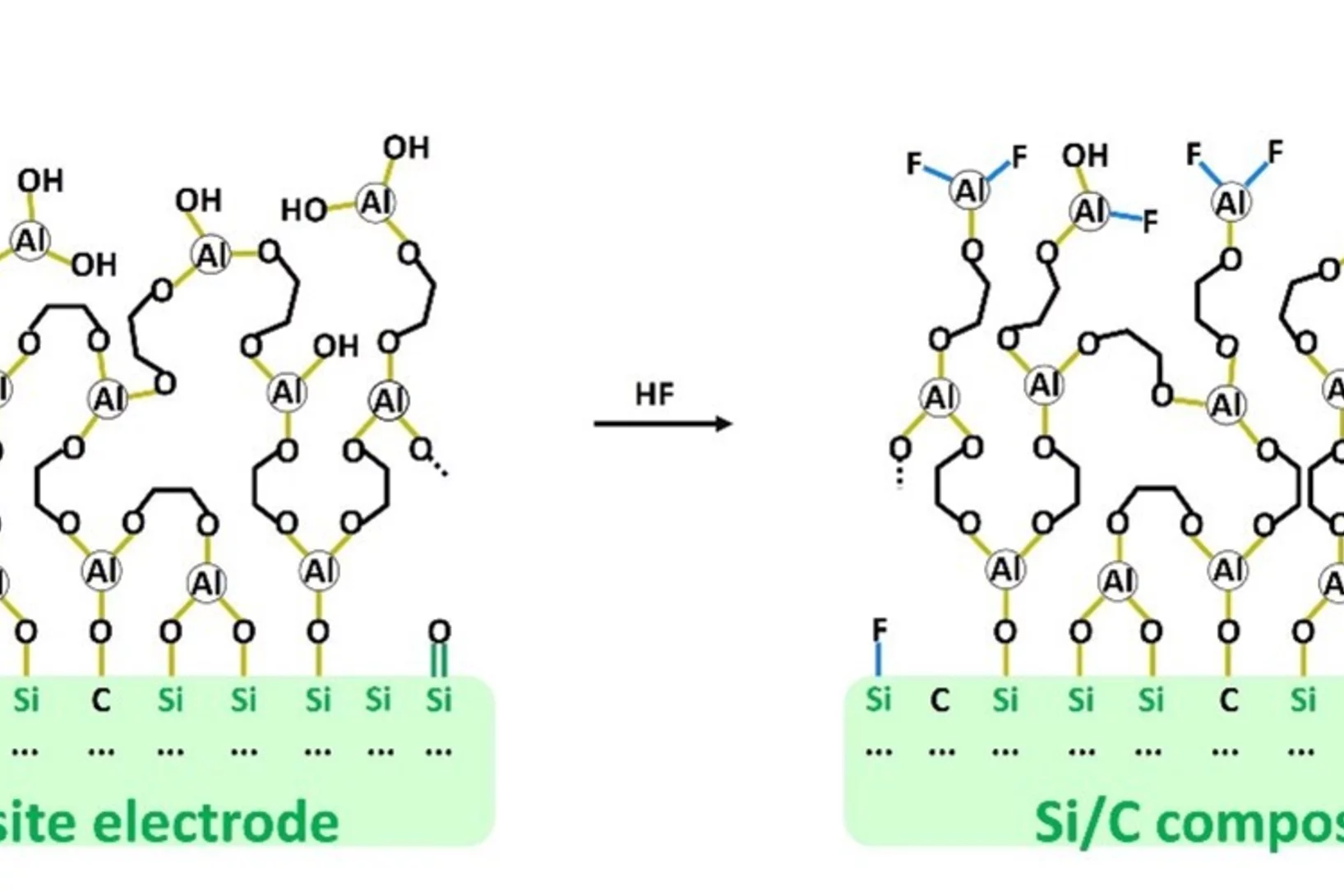
Understanding the Interplay between Artificial SEI and Electrolyte Additives in Enhancing Silicon Electrode Performance for Li-Ion Batteries
Maintaining a stable solid electrolyte interphase (SEI) is crucial for Li-ion battery safety, especially with high-capacity anode containing silicon. Therefore, our study explored long-term cycling of Si electrodes with artificial alucone-based SEI, deposited by molecular layer deposition (MLD) in combination with a fluoroethylene carbonate (FEC) electrolyte additive. MLD of flexible Li-ion permeable artificial SEI coatings onto electrode resulted in improved capacity, enhanced Si electrode cycle life and capacity retention.
Publications
-
Barros Á, Duburg JC, Gubler L, Aranzabe E, Artetxe B, Gutiérrez-Zorrilla JM, et al.
In search of optimal cell components for polyoxometalate-based redox flow batteries: effect of the membrane on cell performance
Energies. 2025; 18(5): 1235 (18 pp.). https://doi.org/10.3390/en18051235
DORA PSI -
Dessiex M, Fischer R, Weng T-W, Marone F, Binder B, Lunati I, et al.
Water management in zero-gap CO2 electrolyzer with forward bias bipolar membranes
Journal of the Electrochemical Society. 2025; 172(5): 054504 (11 pp.). https://doi.org/10.1149/1945-7111/add181
DORA PSI -
Duburg JC, Avaro J, Krupnik L, Silva BFB, Neels A, Schmidt TJ, et al.
Design principles for high-performance meta-polybenzimidazole membranes for vanadium redox flow batteries
Energy and Environmental Materials. 2025; 8(1): e12793 (12 pp.). https://doi.org/10.1002/eem2.12793
DORA PSI -
Dörenkamp T, Zaccarelli A, Büchi FN, Schmidt TJ, Eller J
Guided water percolation in 3D-printed gas diffusion layers for polymer electrolyte fuel cells
ACS Applied Materials and Interfaces. 2025; 17(16): 23959 (13 pp.). https://doi.org/10.1021/acsami.5c00770
DORA PSI -
Giorio C, Borca CN, Zherebker A, D’Aronco S, Saidikova M, Sheikh HA, et al.
Iron speciation in urban atmospheric aerosols: comparison between thermodynamic modeling and direct measurements
ACS Earth and Space Chemistry. 2025; 9(3): 649-661. https://doi.org/10.1021/acsearthspacechem.4c00359
DORA PSI