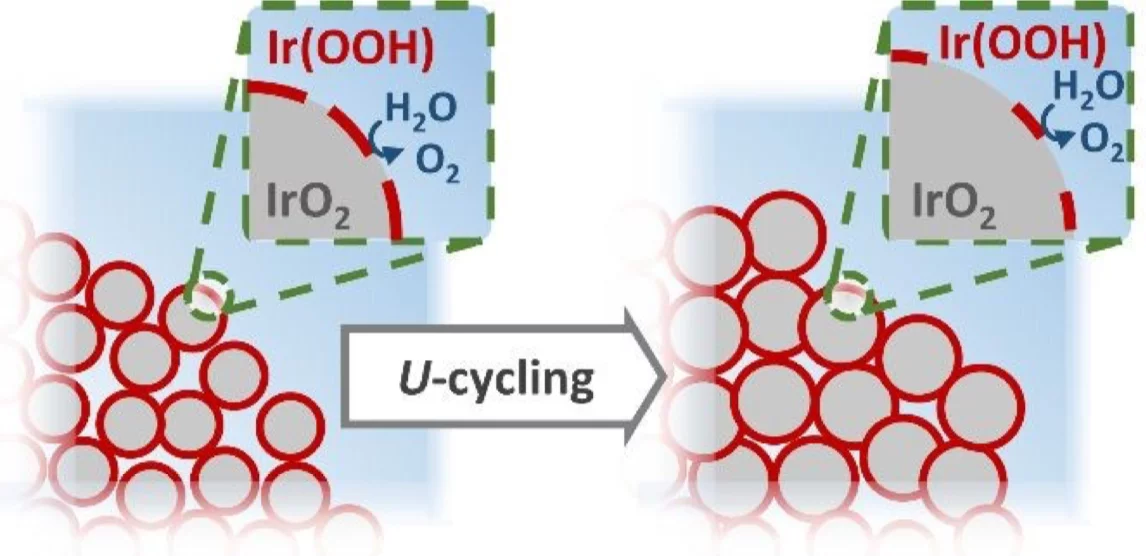
The increasingly popular power-to-gas technology for the utilization of hydrogen as a clean energy vector involves the use of electrolyzers to convert water into H2 and O2. The oxygen evolution reaction (OER) is the least efficient among these processes, and a catalyst is required to speed up its kinetics at the high potentials (customarily ≥ 1.4 V vs. the reversible hydrogen electrode) at which the reaction takes place. The state-of-the-art OER-catalyst is iridium oxide, a material of extremely limited availability in the Earth’s crust and that could therefore jeopardize the development of this technology in the near future. Thus, to minimize the Ir-loading in such electrolyzers, great efforts are being devoted to develop high surface area iridium oxides that achieve maximum OER activity with minimum amounts of Ir but, on the other hand, tend to suffer from a poor operative stability. In order to clarify the mechanism behind this activity loss, in this study two high surface area iridium oxides were characterized under operando conditions using a novel setup that allows the quasi-simultaneous acquisition of anomalous small angle X-ray scattering (A-SAXS) and X-ray absorption spectroscopy (XAS) data. When combined with the results derived from complementing, ex situ characterization techniques (e.g., X-ray photoelectron spectroscopy), the operandoinformation about the catalysts’ morphology and composition inferred from these synchrotron X-ray based techniques allowed to decouple the relative contributions of different instability mechanisms (i.e., Ir-loss, particle agglomeration and/or surface oxidation) to their overall deactivation.
Facility: SLS
Reference: M. Povia et al, Energy and Environmental Science, accepted manuscript (2019)
Read full article: here