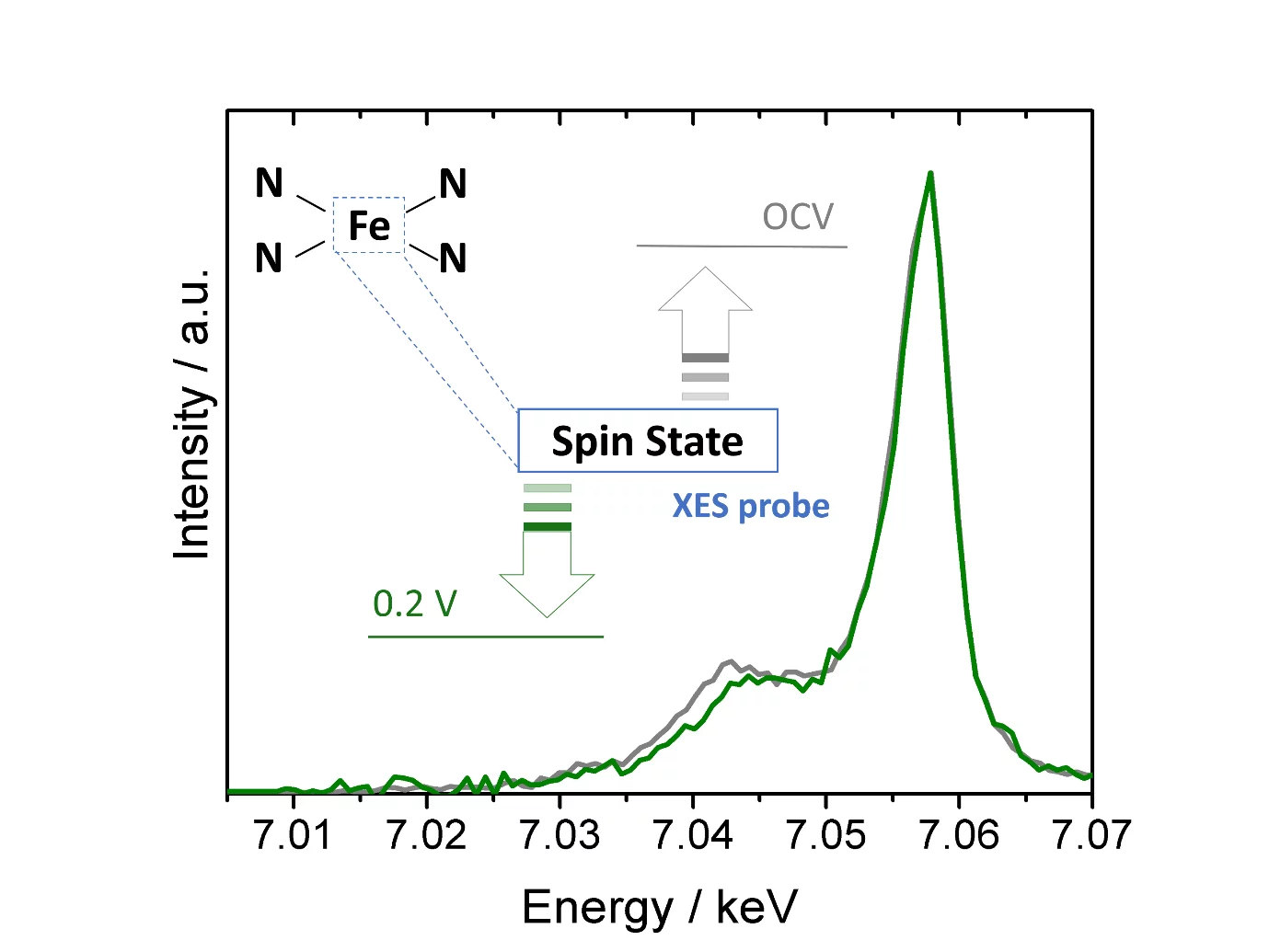
Single atom catalysts hold great promise as O2- or CO2-reduction electrocatalysts, but a deeper understanding of their active sites’ structure and electronic properties is needed in order to render them sufficiently active and stable. To this end, we have used X-ray emission spectroscopy to determine these catalysts’ electronic configuration, and performed in situ measurements that unveil the effect of potential on this key feature.
The rollout of renewable energy conversion technologies like fuel cells and electrolyzers requires inexpensive electrocatalysts for their electrodes’ reactions. In this context, materials featuring single metal atoms coordinated by nitrogen functionalities in a carbon matrix (so called single atom catalysts, or SACs) offer tremendous potential as catalysts for the reduction of O2 or CO2. However, the needed improvements in these materials’ catalytic features (i.e., activity, stability and selectivity) require a better understanding of the structure and electronic properties of their active sites. With this motivation, we have used X-ray emission spectroscopy (XES) to determine the electronic configuration (or spin state) of the active centers in two of these catalysts. To do so, we first collected XES spectra of several compounds with metal coordination environments similar to those of the active sites in SACs and well-defined electronic properties. We then found a correlation between these compounds’ spin states and the intensity of their low energy, Kβ X-ray emission spectral feature (at ≈ 7.04 keV), and used this relation to quantify the catalysts’ average spin state. Finally, we performed additional XES measurements on one of these SACs under electrochemically relevant conditions, and proved for the first time that this average spin state changes as a function of the applied potential. In doing so, our results pave the road for the extended use of XES to study the electronic properties of SACs.
Contact
Dr. Juan Herranz
Senior Scientist, Electrocatalysis and Interfaces Group
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 55 62
E-mail: juan.herranz@psi.ch
Original Publication
Potential‐induced spin changes in Fe/N/C electrocatalysts assessed by in situ X‐ray emission spectroscopy
Viktoriia A. Saveleva, Kathrin Ebner, Lingmei Ni, Grigory Smolentsev, Daniel Klose, Andrea Zitolo, Elena Marelli, Jingkun Li, Marisa Medarde, Olga V. Safonova, Maarten Nachtegaal, Frédéric Jaouen, Ulrike I. Kramm, Thomas J. Schmidt, Juan Herranz
Angewandte Chemie International Edition (2021)
DOI: 10.1002/ange.202016951
Acknowledgement
Swiss National Science Foundation, Ambizione Energy grant nr. PZENP2_173632.