The evolution of O2 occurring in polymer electrolyte water electrolyzer anodes is a very slow reaction that must be catalyzed using iridium (Ir-) based materials. However, Ir is an extremely scarce metal, and thus the extended commercialization of these electrolyzers will only be possible if the amount of Ir implemented in their anodes is drastically reduced. This requires an improved understanding of the individual steps through which these Ir-based materials catalyze the evolution of O2. To shed light on this matter, in this work we studied four different Ir-based catalysts under operative conditions using time resolved X-ray absorption spectroscopy. Our results show for the first time that, despite the differences between these materials, their surfaces must systematically be completely oxidized to a +5 state in order for the evolution of O2 to proceed on them.
Polymer electrolyte water electrolyzers (PEWEs) are very well suited for the production of hydrogen using renewable electricity. However, PEWE-commercialization could be severely limited due to the reliance of this technology on scarce and expensive Ir-based materials to catalyze the evolution of O2 in PEWE-anodes. This translates in the need for a significant decrease of the amount of Ir used in these electrolyzers, which will in turn be possible by improving our understanding of how these materials catalyze the evolution of O2 (i.e., of the so-called reaction mechanism). In this context, there is a vivid debate regarding the changes in oxidation state undergone by the surface of the Ir-based catalysts during the O2-evolution reaction (OER).
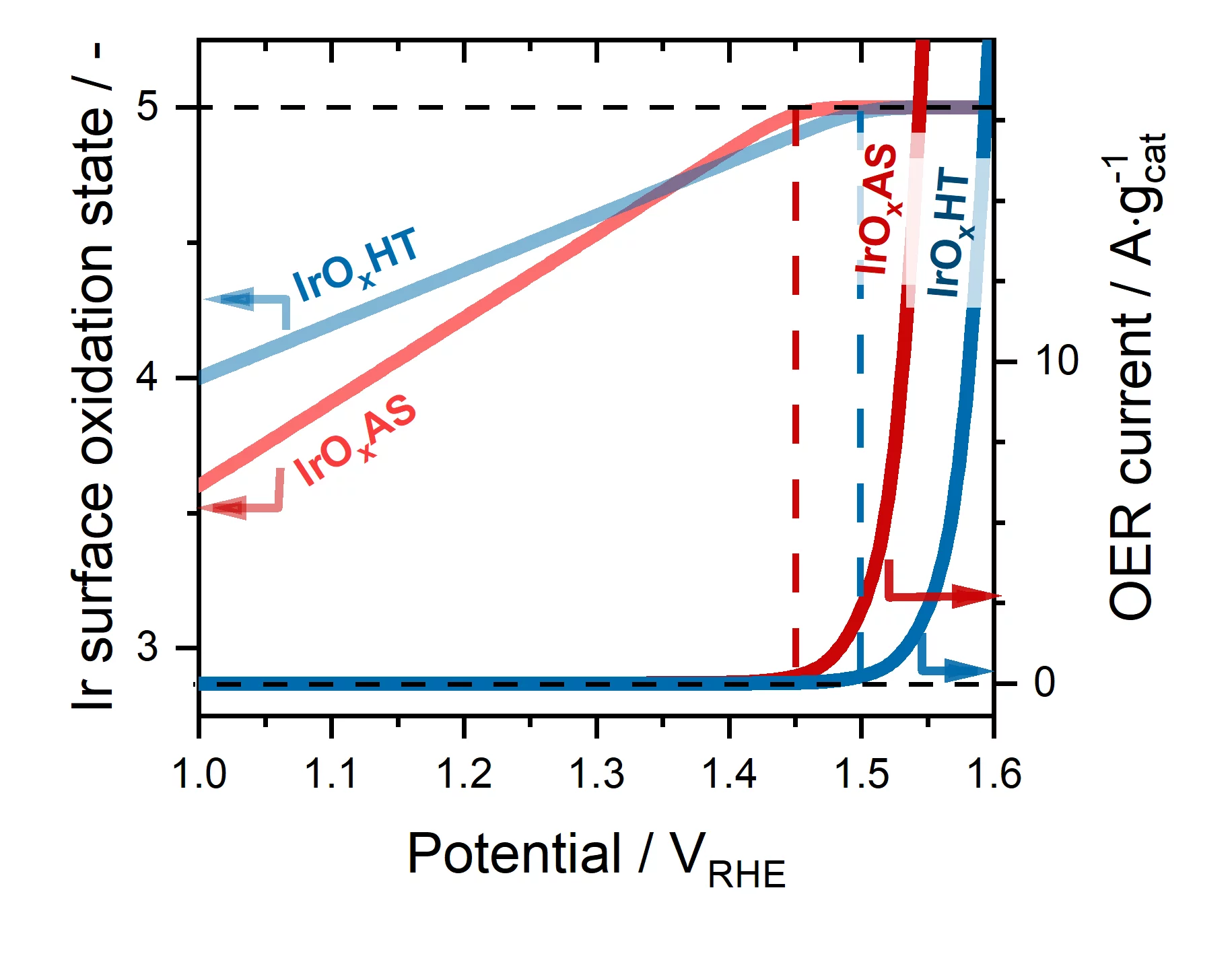
To get a better understanding of this question, in this work we selected four Ir-based catalysts with very different surface areas, initial oxidation states and O2-evolution activities, and studied them under operative conditions using X-ray absorption spectroscopy (XAS). We performed these measurements using a setup available at the SuperXAS beamline of the Swiss Light Source that allowed us to continuously record X-ray absorption spectra with an acquisition frequency of one spectrum per second. The analysis of these time-resolved data showed that the surface of all catalysts becomes linearly more and more oxidized as the applied potential increases. Most importantly, this oxidation process systematically reaches a plateau at a potential specific to each catalyst, but that systematically coincides with the onset of O2-evolution on its surface. Finally, we compared these spectra acquired under operative conditions with others recorded on reference Ir-compounds with well-defined oxidation states; this allowed us to conclude that for all catalysts the surface oxidation state triggering O2-evolution corresponds to Ir+5. As such, our results discard previous reaction mechanisms that postulated that in order to catalyze the OER, Ir-based surfaces only reach oxidation states < +5.
Contact
Dr. Juan Herranz, Senior Scientist
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 55 62
E-mail: juan.herranz@psi.ch
Original Publication
Time-Resolved Potential-Induced Changes in Fe/N/C Catalysts Studied by In Situ Surface Ir+5 formation as a universal prerequisite for O2-evolution on Ir-oxides
Nataša Diklić, Adam H. Clark, Juan Herranz, Dino Aegerter, Justus S. Diercks, Alexandra Beard, Viktoriia A. Saveleva, Piyush Chauhan, Maarten Nachtegaal, Thomas Huthwelker, Dmitry Lebedev, Paula Kayser, José Antonio Alonso, Christophe Copéret, Thomas J. Schmidt
ACS Catal. 13(16), 11069–11079 (2023).
DOI: 10.1021/acscatal.3c01448
Acknowledgement
Swiss Federal Office of Energy and Umicore GmbH & Co KG.