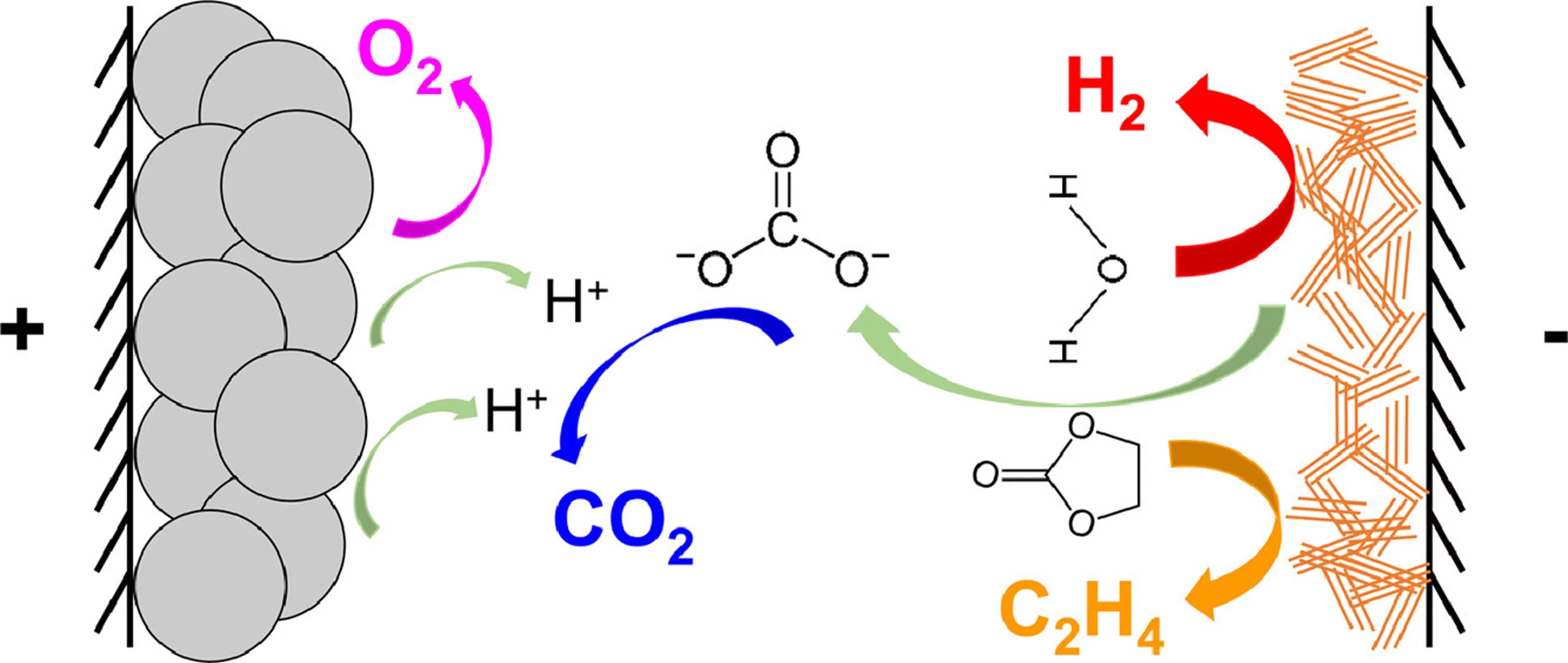
Identification of gaseous decomposition products from irreversible side-reactions enables understanding of inner working of rechargeable batteries. Unlike for Li-ion batteries, the knowledge of the gas-evolution processes in Na-ion batteries is limited. Our study revealed that Na-ion cells develop a less stable solid-electrolyte interphase (SEI) compared to Li-ion cells due to higher solubility of SEI constituents in Na-electrolytes.
Gas-evolution interactions occurring during cell cycling.
Li-ion batteries (LIBs) have been a game-changer for electric vehicles and renewable energy storage, with ongoing improvements making them even better. But now, Na-ion batteries (NIBs) are making a comeback: first explored in the 1980s, NIBs are gaining attention as a more geopolitically independent alternative to LIBs. Another big advantage of NIBs is that they can use aluminum (Al) as the anode current collector instead of the heavier, more expensive copper (Cu) used in LIBs, helping to reduce manufacturing costs. In addition, the chemistry of sodium (Na) is quite similar to lithium (Li), allowing scientists to quickly apply what they’ve learned from LIBs to NIBs, and also enabling manufacturers to use the same production lines, removing large economic hurdles form NIB commercialization.
However, not everything from LIBs can be directly applied to NIBs. Sodium is more reactive than lithium, which affects the stability of the solid-electrolyte interphase (SEI) – a crucial layer that forms on the anode and protect electrolyte for further decomposition. In NIBs, the SEI is less stable because Na-based components dissolve more easily, leading to more electrolyte degradation. Because metallic sodium is so reactive, half-cells (where counter electrode is Na-metal) can have more issues with side-reactions and gas production. Therefore, it iss important to test NIBs electrode materials in full-cells, especially when long-term performance is crucial.
To understand and tackle these stability issues, we have been studying the gases released during battery operation, which helps to understand the degradation mechanisms of Na-ion batteries better. The use of a technique, called online electrochemical mass spectrometry (OEMS), to monitor these gases in real-time allows understanding of the NIBs uniqueness and helps developing dedicated recipes for appropriate testing as well as their long-term service.
In our study, we showed that real-time gas analysis is a unique way to study battery chemistry. However, it doesn’t detect side-reactions that don’t produce gas. To get a complete understanding of what’s happening, we need to use other techniques that look at the bulk material (like transmission electron microscopy), the surface (like X-ray photoelectron spectroscopy), and the electrolyte solutions (like NMR and LC-MS). This combined approach would give us a full picture of the interactions at the Li-ion and Na-ion battery’s interfaces.
Contact
Dr. Sigita Trabesinger
Head of Battery Electrodes and Cells Group
Paul Scherrer Institute PSI, 5232 Villigen PSI, Switzerland
Telephone:+41 56 310 5775
E-mail: sigita.trabesinger@psi.ch
Original Publication
Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry
Authors: Leiting Zhang, Chrysi Tsolakidou, Sathiya Mariyappan, Jean-Marie Tarascon, Sigita Trabesinger
Energy Storage Materials, (2022) 141547.
DOI: https://doi.org/10.1016/j.ensm.2021.07.005
Acknowledgement
The financial support from the Swiss Competence Center for Heat and Electricity Research (SCCER–HaE) is greatly appreciated. C. T. thanks the International Association for the Exchange of Students for Technical Experience (IAESTE) for giving her a chance to conduct research at PSI.