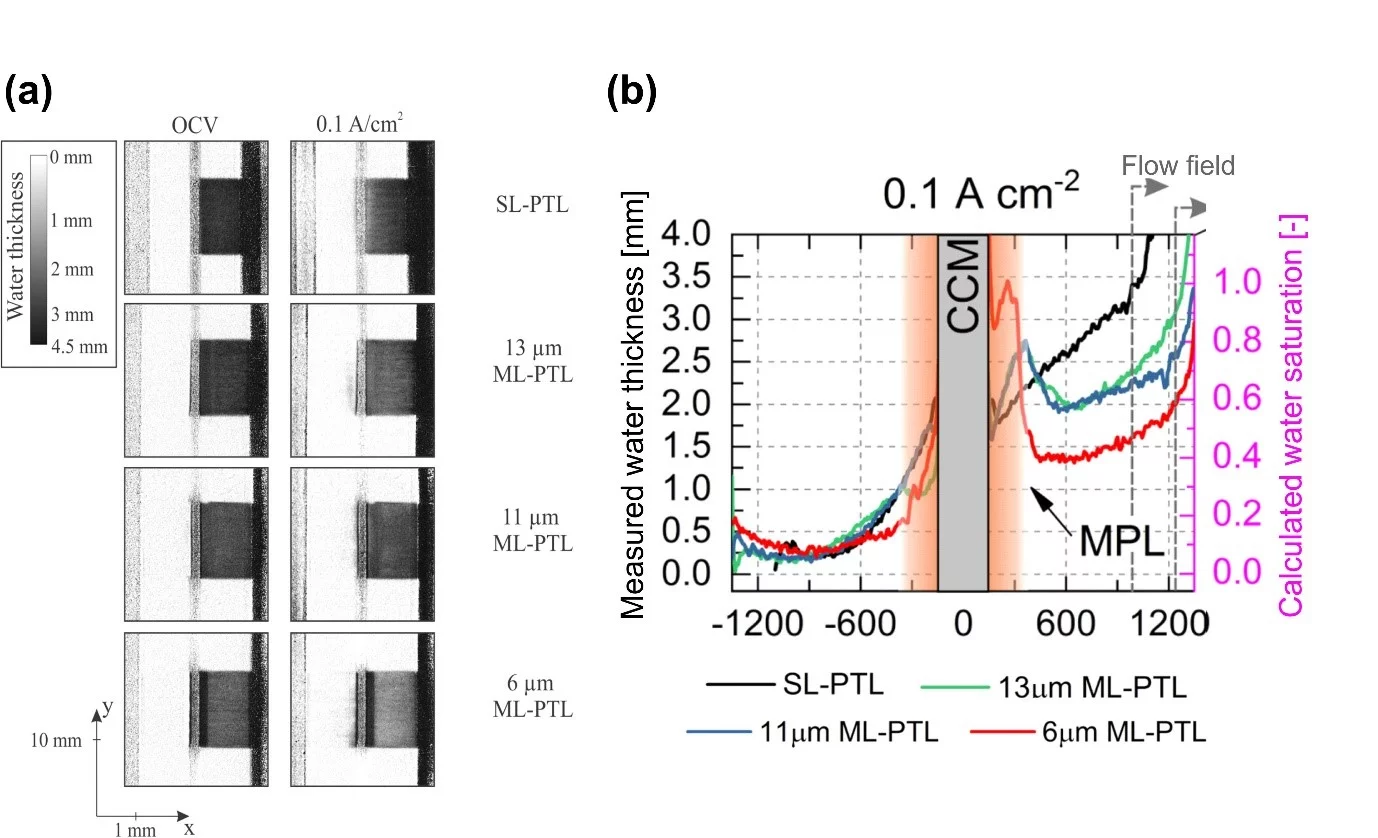
Polymer Electrolyte Water Electrolyzers (PEWE), due to their excellent dynamic characteristics, can provide an economical solution to the intermittent nature of new renewable sources, by converting the excess electricity into hydrogen. However, improvements in efficiency and in capital cost are still required for the large-scale deployment of this solution. In this context, we studied whether the efficiency improvements observed when using porous structures featuring a micro-porous layer (MPL) can be attributed to a better distribution of the water.
In fact, the water supplied and the gas produced by the electrochemical reaction share the same pathway, resulting in a so-called two-phase flow phenomena. If the volume occupied by the gas hinders the supply of water, this can have a detrimental impact on efficiency. To elucidate the impact of adding a MPL– an additional layer with a similar porosity, but a finer grain size as the bas porous structure –, we used high resolution neutron radiography to measure the water and gas distribution across the structure of operating PEWE cells. An important characteristic of this method is that it provides an important contrast for water, while the neutron beam can penetrate through large thicknesses of relatively dense materials. The conducted measurements demonstrated that the presence of a MPL has a significant impact on the water and gas distribution. Near the catalyst layer where the reaction takes place, a larger water saturation is observed – meaning a much lower fraction of the pores is occupied by gas – than when no MPL is used. This effect is particularly strong when using the MPL with the smallest pore size (6 µm). On the other hand, using a MPL results is a much lower water saturation (higher gas fraction) in the bulk of the coarse porous layer. The strong contrast in saturation between the MPL and the region with coarser pores can be explained by the fact that that each gas transport pathway through the small pores of the MPL emerges in a large pore, resulting in a significant increase of the volume occupied by gas at this interface. The observation that the presence of a MPL strongly affects the water distribution in a positive way near the electrode may lead to conclude that the improved water supply is responsible – at least in part – for the improvement of performance observed when using MPLs. However, this hypothesis is put in perspective by the fact that the magnitude of the impact of MPL on water distribution is a function of the MPL pore size, with a stronger impact for lower pore sizes. On the other hand, the performance improvement is observed when using a MPL, independently on the pore size. Therefore, another explanation for the performance improvement is that the MPL provides a closer contact to the electrode, removing the dependency on the ionic conductivity of the catalyst layer. Finally, it is remarkable that the performance improvement when using a MPL occurs despite a very strong increase of the gas saturation in the coarse part of the porous layer. Obviously, the remaining water pathways in this case are sufficient to supply the electrochemical reaction without detrimental effects. This highlights the fact that an important potential remains for more economical structures for water supply and gas removal, as long as the interface to the catalyst layer is carefully designed.
Contact
Dr. Pierre Boillat
Head of Neutron Imaging of Electrochemical Systems Group
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 27 43
E-mail: pierre.boillat@psi.ch
Original Publication
Elucidation of fluid streamlining in multi-layered porous transport layers for polymer electrolyte water electrolyzers by operando neutron radiography.
M. Zlobinski, T. Schuler, F.N. Büchi, T.J. Schmidt, P. Boillat
J. Electrochem. Soc. 168, 014505 (2021)
DOI: 10.1149/1945-7111/abcf19
Acknowledgement to Funding Agency
The authors would like to thank the Swiss Federal Office of Energy (SFOE, for Grant Nos. SI/501331‐01 and SI/8100066). T.J. Schmidt thanks Innosuisse and the Swiss Competence Center for Energy Research (SCCER) Heat & Electricity Storage.