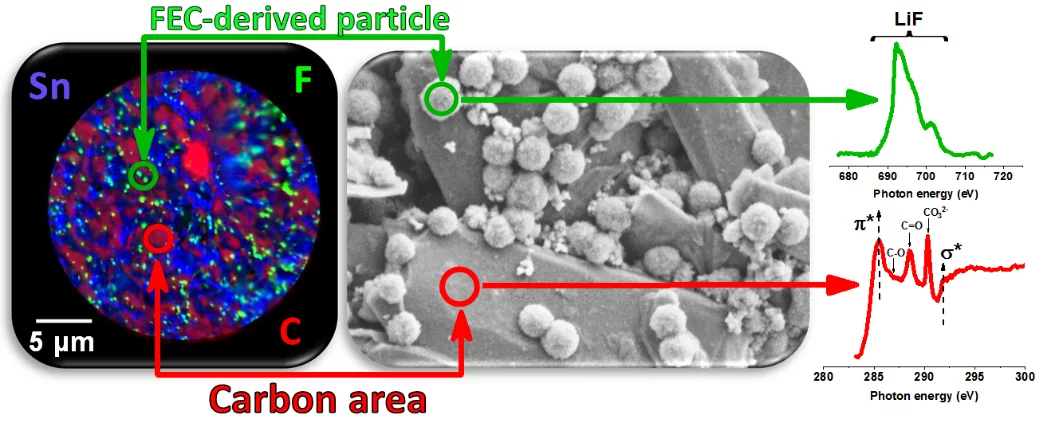
Fluoroethylene-carbonate is often referred to as a film-forming electrolyte additive for Li-ion batteries, resulting in high quality Solid–Electrolyte-Interphase on negative electrode, however, the underlying mechanism, even if thought to be known, has been only clarified due to our targeted experimental design, combining systematic electrochemical, chemical and microscopy characterization techniques. We have shown that first the formation of inorganic LiF-rich particles appear and only later the carbonate-rich film is actually formed.
High energy density is one of the clear trends in future battery technologies, especially in portable devices and mobility sectors. Energy density of Li-ion cells can be increased by using materials with higher capacity and by extending operating voltage window of the cell. Despite the fact that positive electrodes have the lower specific capacities than then negative one, the increase of negative electrode capacity also leads to significant improvement of energy density. Besides the most-commonly used graphite, there is a number of negative electrode active materials, whose capacity is many times higher than that of graphite, such as silicon, tin oxide and others. However, these materials often exhibit the volume expansion upon lithiation and therefore an SEI formed on their surface is unstable. The most popular strategy for improving their cycling stability is the use of electrolyte additives that are reduced at the potentials more positive than electrolyte solvents. Their decomposition products are incorporated in the SEI, leading to a more stable electrode/electrolyte interface. One of the most-investigated electrolyte additives is fluoroethylene carbonate (FEC), because it has been shown to lead to a significant performance enhancement of Si- and Sn-based anodes. Many reaction pathways have been proposed but there is currently no agreement on the exact type of chemical compounds constituting the decomposition products, as well as on the mechanism of FEC decomposition.
For this reason we embarked the systematic study, tracking morphological and chemical changes during electrochemical decomposition of FEC, and found that despite FEC often being referred as film-forming additive, in the first stage of its decomposition, spherical particles, mainly consisting of lithium fluoride are formed. And only later the carbonate-rich film covers the entire electrode, including the LiF-rich spheres. This phenomenon has been overlooked by researchers earlier due to very simple reason—the imaging by electrons, using SEM, is often done at high accelerating voltages, i.e. 10–20 kV, as it allows simultaneous EDX analysis, at least in older generation of characterization tools. At this acceleration voltages, the LiF particles become invisible, as is shown in the supplementary information of our publication. And only acceleration voltages below 5kV allow to visualize the spherical particles of LiF, formed by FEC reduction.
We have also found that the size and amount of particles strongly depend on the testing cell medium, where electrolytes with higher dielectric constant lead to larger particle size, as do the high-capacity active electrode materials, both contributing to final properties of FEC-derived SEI. The results of this study provide a deeper understanding of how fluorine additives work and enables tuning of the SEI properties by using laws of simple crystal-growth theory to fit the conditions leading to the desired morphological and chemical outcome. This is an important step to enabling the rational design of fluorine-rich electrolytes and fluorine-based sacrificial additives towards the stabilization of high-specific-capacity anodes and, consequently, of batteries with high energy density.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publications
Evidence for Stepwise Formation of Solid Electrolyte Interphase in a Li-ion Battery
Yuri Surace, Daniela Leanza, Marta Mirolo, Lukasz Kondracki, C.A.F. Vaz, Mario El Kazzi, Petr Novák, Sigita Trabesinger
Energy Storage Materials 44, 156-167 (2022).
DOI: 10.1016/j.ensm.2021.10.013
Acknowledgement
InnoSuisse (Swiss Innovation Agency, project number 18254.2) and PSI CROSS initiative are acknowledged for financial support. Part of the work was performed at the Surfaces/Interfaces Microscopy (SIM) beamline of the Swiss Light Source (SLS), Paul Scherrer Institute (PSI).