PhD student Hao Guo received the SAPhW Poster Award for Best Poster in Clinical Pharmacy at the 2025 Swiss Pharma Science Day
We congratulate Hao Guo for receiving the award of the best poster at the Swiss Pharma Day.
Mit Terbium gegen Lymphdrüsenkrebs
Vielversprechende Laborexperimente am PSI zeigen: EineRadionuklidtherapie mit dem radioaktiven Element Terbium könnte Lymphdrüsenkrebs wirksam bekämpfen.
Dr. Yingfang He has been honored with the Alavi-Mandell Award 2025
We congratulate Dr. Yingfang He for the excellent research work she did during her time at the Center for Radiopharmaceutical Sciences.
Meilenstein der Superlative
PSI-Spin-off Araris Biotech AG erreicht Bewertung auf Unicorn-Level!
IMPACT für die Schweizer Gesellschaft
Weltspitze bei den Myonen und in der Herstellung medizinischer Radionuklide: Die weitreichende Bedeutsamkeit des geplanten Upgrades.
Dr. Chiara Favaretto has been honored with the Alavi-Mandell Award 2024
We congratulate Dr. Chiara Favaretto for the excellent research work she did during her time at the Center for Radiopharmaceutical Sciences.
The Executive Committee on Personalized Health and Related Technologies (PHRT) has supported the PROGNOSTIC translational Project with a Grant of CHF 2 million
The translational project "PROGNOSTICS", proposed by a consortium led by Prof. Roger Schibli, received CHF 2 million funding from the PHRT initiative of the ETH Domain(https://www.sfa-phrt.ch/two-clinical-trial-projects-launched-transforming-clinical-care-with-eth-technologies/). The study is being conducted with the Co-applicants Prof. Dr. med. Damian Wild at the University Hospital Basel and Prof. Dr. Nicola Aceto of the ETHZ. The consortium will test a new radiopharmaceutical developed at the Center for Radiopharmaceutical Sciences in 36 patients with advanced prostate cancer.
ETH Rat promotes PD Dr. Cristina Müller to the rank of a Titular Professor of the ETH Zurich
Professor Dr. Cristina Müller.
Prof. Roger Schibli was awarded an Honorary Fellow of the Society of Radiopharmaceutical Sciences (SRS)
Prof. Roger Schibli was awarded an Honorary Fellow of the Society of Radiopharmaceutical Sciences (SRS) “in recognition of substantial contribution to the SRS”.
Prof. Roger Schibli is selected a Board Member of the Society of Radiopharmaceutical Sciences
Prof. Roger Schibli (third person from the left).
IPW Young Investigator Award 2022
Dr. Francesca Borgna, former Marie Curie Fellow at the Center for Radiopharmaceutical Sciences awarded by the Institute of Pharmaceutical Sciences in 2022 and gave the IPW Young Scientist lecture entitled: "Combination of Terbium-161 with Somatostatin Receptor Antagonists: a Potential Paradigm Shift for the Treatment of Neuroendocrine Neoplasm.
Poster Prize 2022: First Prize (AKB Foundation) of the SAPhW Poster Award at the Swiss Pharma Science Day 2022
Rahel Wallimann, PhD student in the “Nuclide Chemistry Group”, received the first prize (AKB Foundation) of the SAPhW Poster Award at the Swiss Pharma Science Day 2022.
NMB/Eckelman Young Investigator Award 2022
Chiara Favaretto, PhD student in the “Radionuclide Development” group at the Center for Radiopharmaceutical Sciences, received the NMB/Eckelman Young Investigator Award for the abstract entitled: “Production and radiochemical separation of terbium-155 from enriched gadolinium target material and its preliminary application in SPECT imaging”, presented at the International Symposium on Radiopharmaceutical Sciences (iSRS 2022).
Terbium Triumph
Bench-to-bedside successes: Fruitful collaborations at PSI’s Center for Radiopharmaceutical Sciences make bringing Terbium-161 to the clinic a reality.
Effektive kombinierte Tumortherapie
Zwei gemeinsam eingesetzte Präparate verringern das Wachstum von Krebsgewebe.
Presentation Prize 2021 (Doktorandentag, Institute of Pharmaceutical Sciences, ETH Zurich)
Luisa Deberle, PhD student in the “Nuclide Chemistry Group” received the price for the best oral presentation
EANM Marie-Curie Award 2021 went to VIVIANE TSCHAN
Viviane Tschan received this year’s Marie-Curie Award for a new concept of radioligand therapy of prostate cancer.
Neuartige und kommende medizinische Radionuklide
Bessere Behandlung von streuenden Tumoren.
A strong commitment for science communication
PSI is engaging in public outreach at a major event in Zürich, the "Scientifica" Science Days September 4 and 5.
Prof. Dr. Roger Schibli has been elected a Council Member 2020-2022 of the European Society for Molecular Imaging - ESMI
European Society for Molecular Imaging - ESMI
The ESMI represents and advocates IMAGING SCIENCE
The ESMI is providing an international, interdisciplinary platform for knowledge exchange in the field covering basic sciences, translational aspects as well as clinical applications.
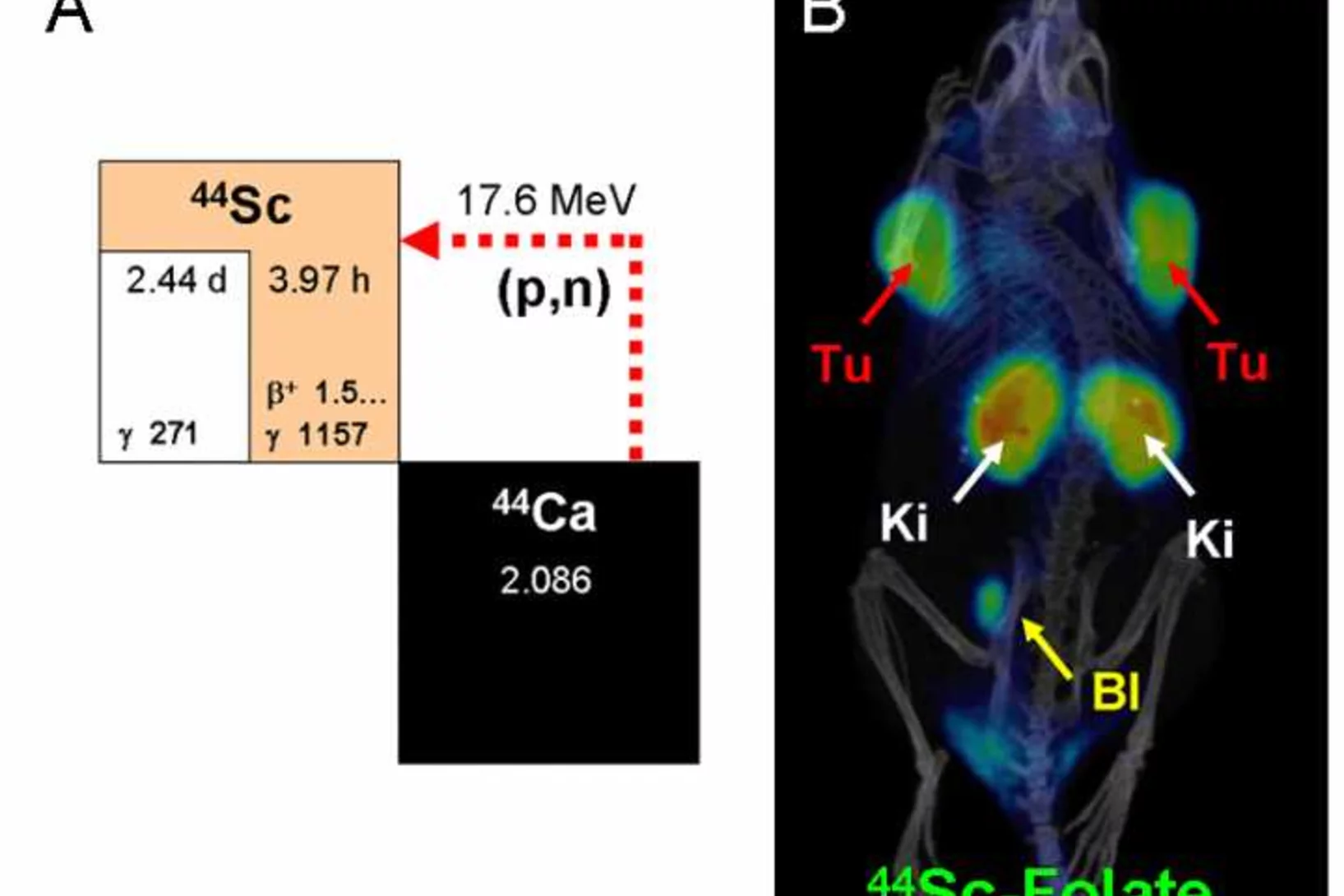
Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent beta - emitters: In vitro and in vivo study of a 44Sc-DOTA-folate conjugate
Research Division Biology and Chemistry (BIO), Folate Receptor Targeting Group, Head Cristina Müller. In recent years, implementation of 68Ga-radiometalated peptides for PET imaging of cancer has attracted the attention of clinicians. Herein, we propose the use of 44Sc (half-life = 3.97 h, average β+ energy [Eβ+av] = 632 keV) as a valuable alternative to 68Ga (half-life = 68 min, Eβ+av = 830 keV) for imaging and dosimetry before 177Lu-based radionuclide therapy.