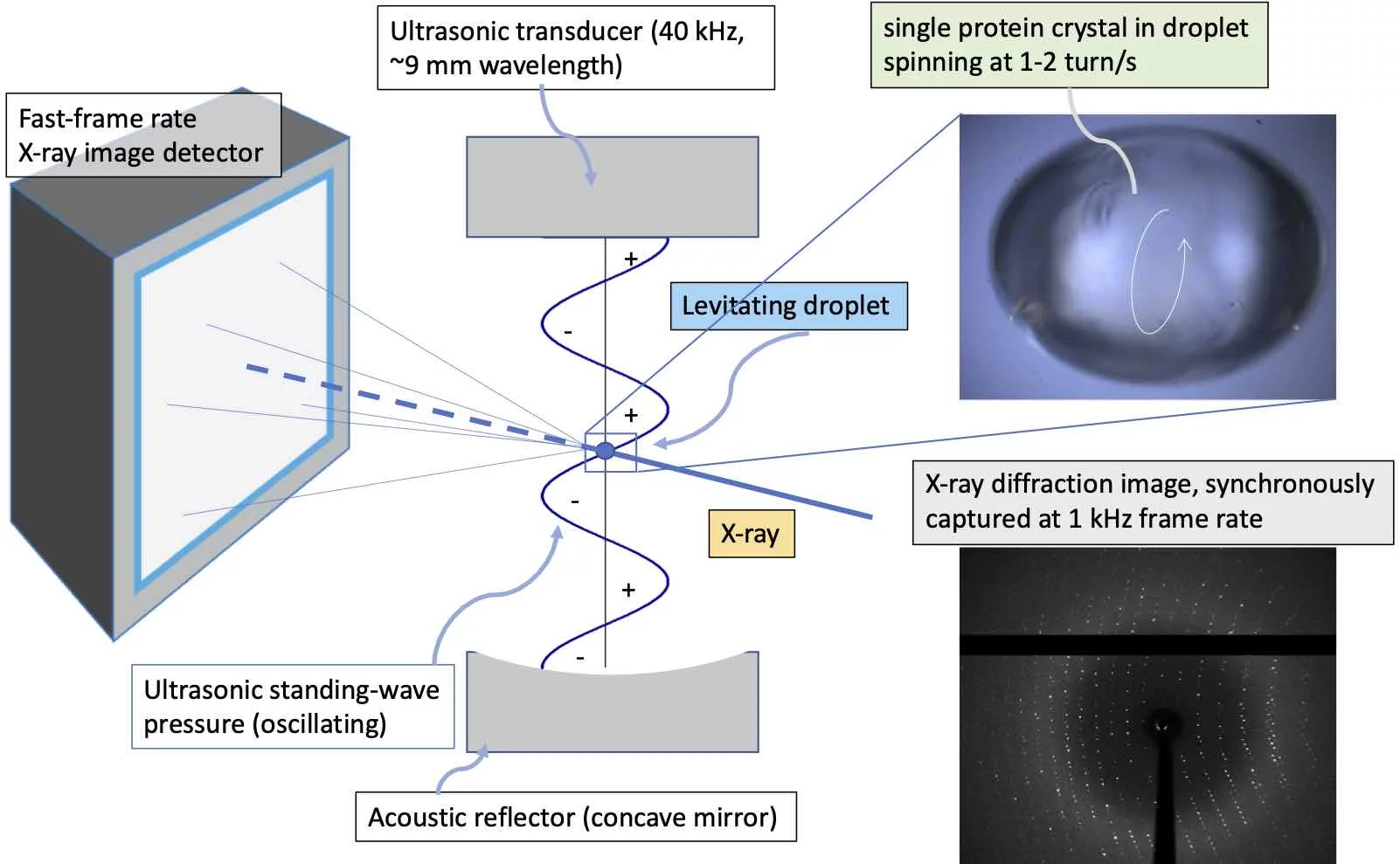
Room-temperature crystallography, no mounts needed: our ultrasonic acoustic-levitation diffractometer (ALD) rotates microcrystals inside levitated droplets and records continuous diffraction with a fast pixel detector—enabling high-speed, sample-efficient, high-throughput measurements and ~0.1 s solution mixing for time-lapse studies.
See Ultrasonic acoustic levitation for fast frame rate X-ray protein crystallography at room temperature(link is external) and highlight, "Acoustic levitation and rotation of thin films and their application for room temperature protein crystallography,", and the Scientific Highlight of PSD and BIO/LNB with a video showing how easy to load these films onto an acoustic levitator.
Ultrasonic acoustic-levitation diffractometer (ALD): room-temperature structures at speed
We’re developing a protein-crystallography workflow that keeps samples off solid supports. The ALD combines ultrasonic acoustic levitation, a high-brightness X-ray beam, and a fast, pixelated detector to capture diffraction while microcrystals freely reorient in a levitated droplet. The platform aims for high speed, high sample efficiency, and true high throughput—all at room temperature. Development is underway at the PXI (XSA06) beamline of the Swiss Light Source.
How it works
- A focused X-ray beam irradiates a droplet stably levitated by ultrasound.
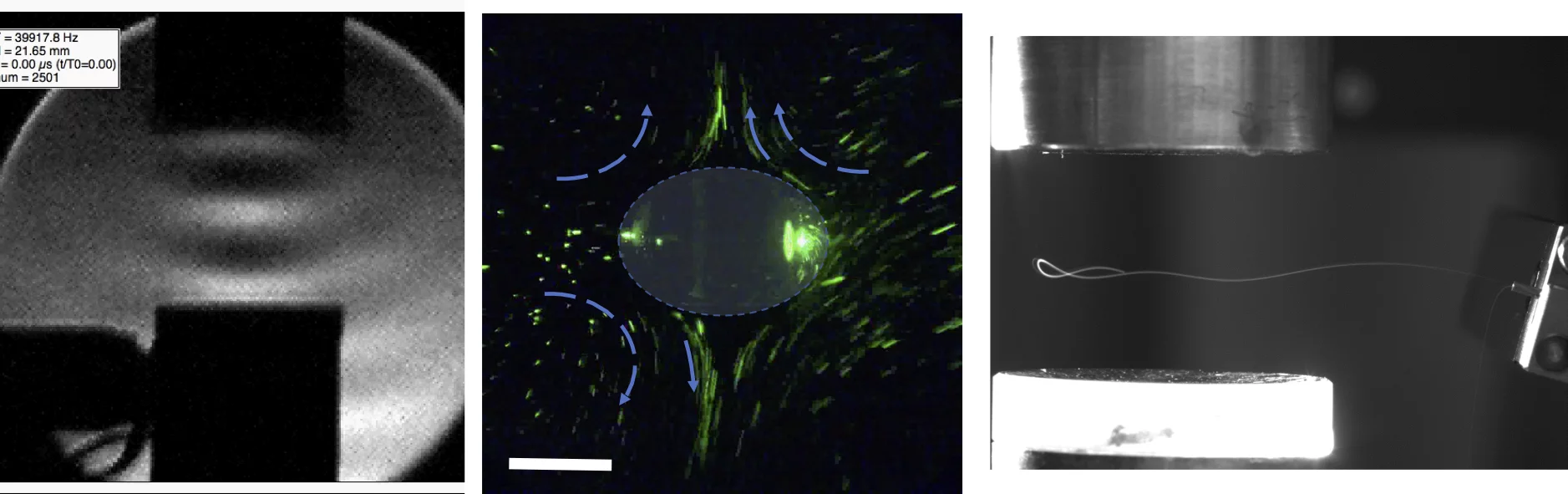
- Acoustic streaming induces controlled rotation/tumbling of microcrystals, sweeping reciprocal space continuously.
- A fast-frame-rate detector records diffraction images as orientations evolve, enabling structure solution without mounting.
What’s new in our approach
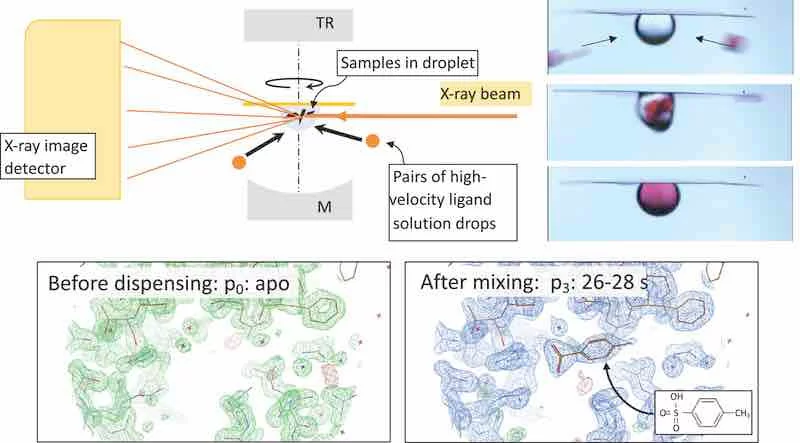
- Fast solution exchange: inertial (ultrasound-driven) mixing reduces reagent exchange times to ~0.1 s in sub-µL to few-µL droplets—suitable for time-lapse and rapid-onset ligand binding.
- Continuous orientation sampling: acoustic streaming provides a natural, contact-free rotation, avoiding mechanical goniometers and reducing handling steps.
- Sample-efficient: no loops, meshes, or pins; droplets serve as carriers, minimizing losses and simplifying cleanup.
Why this matters
- Room-temperature relevance: dynamics and ligand interactions can differ from cryo; ALD lets us watch them closer to native conditions.
- Throughput & automation: stable levitation + fast detectors + scripted delivery/mixing pave the way for routine screening and time-resolved studies.
- Gentle handling: acoustic forces hold and move crystals without touching them—handy for fragile specimens and soft-matter systems.
Where we’re headed next
- Robust, microscope-mountable levitator stages for day-to-day operation.
- Integrated microfluidic delivery for precise dosing and extraction.
- Standardized mix-then-probe protocols for ligand binding and conformational changes.
Collaboration & support
This work is carried out with the Center for Photon Science , and is supported by the Swiss National Science Foundation, Innosuisse (in collaboration with leadXpro AG), and the EU Horizon 2020 programme.
References
[1] Soichiro Tsujino, Takashi Tomizaki, Ultrasonic acoustic levitation for fast frame rate X-ray protein crystallography at room temperature, Scientific Reports, 6, Article number: 25558 (2016); https://doi.org/10.1038/srep25558
[2] Soichiro Tsujino, Akira Shinoda and Takashi Tomizaki, On-demand droplet loading of ultrasonic acoustic levitator and its application for protein crystallography experiments, Applied Physics Letters, 2019, Volume 114, Number 21, Page 213702; DOI: 10.1063/1.5095574
[3] Soichiro Tsujino, Yohei Sato, Yasushi Takeda and Takashi Tomizaki, Oscillation resonances and anisotropic damping of the motion of acoustically levitated droplets in single-axis acoustic levitators, Applied Physics Letters, 2019, Volume 115, Number 5, Page 053702; DOI: 10.1063/1.5112109
[4] Takashi Tomizaki, Akira Shinoda and Soichiro Tsujino, Single crystal time-lapse measurement using ultrasonic acoustic levitation 2019; DOI: 10.1063/1.5084703
[5] Soichiro Tsujino and Takashi Tomizaki, Applications of Acoustic Levitation in Chemical Analysis and Biochemistry, in Acoustic Levitation (Nature Springer, 2020); ISBN : 978-981-32-9064-8; DOI: 10.1007/978-981-32-9065-5_9
[6] Michal. W. Kepa, Takashi Tomizaki, Yohei Sato, Dmitry Ozerov, Hiroshi Sekiguchi, Nobuhiro Yasuda, Koki Aoyama, Petr Skopintsev, Jörg Standfuss, Robert Cheng, Michael Hennig & Soichiro Tsujino, Acoustic levitation and rotation of thin films and their application for room temperature protein crystallography, Scientific Reports volume 12, Article number: 5349 (2022); https://doi.org/10.1038/s41598-022-09167-z
[7] Shichao Jia, Yohei Sato, Soichiro Tsujino, Size and shape dependent rotation characteristics of thin film ultrasonic rotors, Appl. Phys. Lett. 121, 254102 (2022); DOI: https://doi.org/10.1063/5.0126000
[8] Soichiro Tsujino, Yohei Sato, Shichao Jia, Michal W. Kepa, Sofia Trampari, Takashi Tomizaki, Inertial mixing of acoustically levitated droplets for time-lapse protein crystallography, Droplet, 28 May 2024; DOI: https://doi.org/10.1002/dro2.132