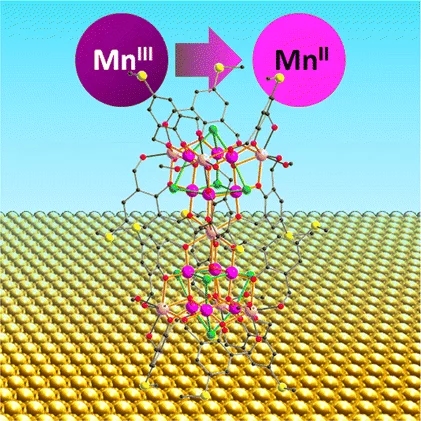
The magnetic properties of a Mn19 coordination cluster equipped with methylmercapto substituents on the organic ligands, [MnIII12MnII7(μ4-O)8(μ3-Cl)7.7(μ3-OMe)0.3(HLSMe)12(MeOH)6]Cl2 27MeCN(Mn19(SMe))(H3LSMe = 2,6-bis(hydroxymethyl)-4-mercaptomethylphenol) deposited on Au(111) surfaces from solution, have been investigated by X-ray absorption spectroscopy and X-ray magnetic circular dichroism. The data reveal that in the submonolayer regime the molecules contain only divalent MnII in contrast to the presence of MnII and MnIII ions in the powder sample. Brillouin function fits to the field-dependent magnetization suggest that the total spin ground state in the submonolayer is much lower than STOT = 83/2 of the pristine molecules. These findings suggest that significant changes of the electronic structure, molecular geometry, and intramolecular exchange coupling take place upon surface deposition. A sample with coverage of 2-3 monolayers shows the presence of MnIII, suggesting that a decoupling layer could stabilize the Mn19 core on a metallic surface.
Original Publication
Reduction of Mn19 Coordination Clusters on a Gold SurfaceJ. Dreiser, A. M. Ako, C. Wäckerlin, J. Heidler, C. E. Anson, A. K. Powell, C. Piamonteze, F. Nolting, S. Rusponi, and H. Brune
J. Phys. Chem. C, 2015, 119 (7), pp 3550-3555 (Publication Date Web: January 13, 2015), DOI:10.1021/jp510240b
Contact
Dr Jan DreiserLaboratory for Synchrotron Radiation - Condensed Matter Physics
Paul Scherrer Institute, 5232 Villigen, Switzerland
Telephone: +41 56 310 - 5895, email: jan.dreiser@psi.ch