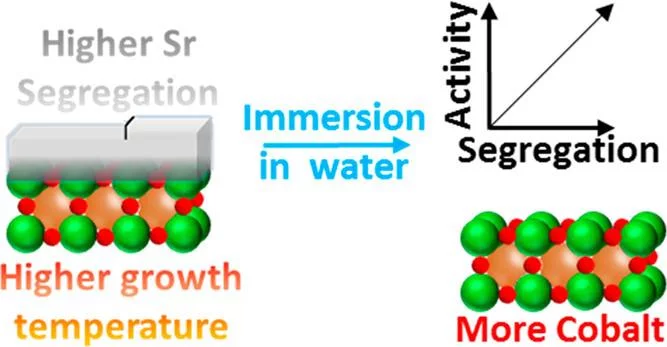
La1–xSrxCoO3-δ perovskites are potential catalysts for the anodic reaction of alkaline water electrolyzers, i.e., the oxygen evolution reaction (OER). It is well-known that La1–xSrxCoO3−δ perovskites can easily display strontium surface segregation, but how this influences the performance of La1–xSrxCoO3−δ perovskites as anodic electrode in alkaline water electrolyzers, particularly in terms of OER activity, has not been unveiled yet. This study focuses on La0.2Sr0.8CoO3−δ, which shows relatively high activity for the OER, and reveals the influence of the preparation temperature on the amount and morphology of segregated strontium-containing islands. Thin film samples were prepared at different temperatures by using pulsed laser deposition. Those samples were then characterized with synchrotron-based X-ray photoelectron spectroscopy “as prepared” and after being immersed in ultrapure water. We found that higher preparation temperatures enhance the segregation of strontium, which is then almost quantitatively removed by washing the samples with ultrapure water. After immersion in water, the samples expose a cobalt-rich surface. Investigating the OER activity as a function of the perovskite deposition temperature, it has been found that the higher the deposition temperature (i.e., the more extended the strontium segregation), the higher the OER activity. Such an effect has been linked to the higher amount of cobalt accessible after removing the strontium segregated islands.
Facility: TFI, ENE, SLS, ETHZ
Reference: A. Boucly et al., Chem. Mater. 32, 5256−5263 (2020)
Read full article: here