In diabetes and aging, glycation of proteins is a phenomenon of non-enzymatic nature and thus not easily controlled. Glycation of collagen distorts its structure, renders the extracellular matrix stiff and brittle and at the same time lowers the degradation susceptibility thereby preventing renewal.
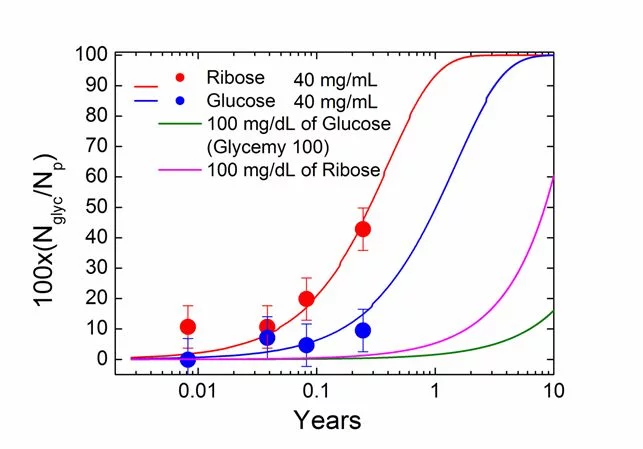
Based on models described in our latest paper and with parameters determined with picometer precision from our experimental small and wide‐angle X‐ray scattering (SAXS/WAXS) imaging data, we describe the glycation of type 1 collagen in bovine pericardium derived bio-tissues upon incubation in glucose and ribose. With arginine and lysine/hydroxylysine amino acids as the primary sites of glycation and assuming that the topological polar surface area of the sugar molecules determines the glycation rates, we model the glycation as a function of time and determine the glycation rate and thus progression of the glycation as well as the resulting volume increase.
As an outlook, our model with refined parameters may be used to determine an estimation of the glycation state for realistic progressions of blood sugar concentrations occurring in healthy and diabetic subjects. This glycation state could be verified against experimental data, taking available information on the vastly varying renewal rates of collagen in its various functions in the body into account. We hope that in this way a better understanding of glycation is reached and that the experimental model system used for determining the experimental data for this study could potentially be validated as testbed for glycation and diabetes, a testbed without the need of sacrificing animals for this purpose.
Contact
Dr. Oliver Bunk
Paul Scherrer Institut
E-mail: oliver.bunk@psi.ch
Dr. Cinzia Giannini
Istituto di Cristallografia
Consiglio Nazionale delle Ricerche
E-mail: cinzia.giannini@ic.cnr.it
Original Publications
Liberato De Caro, Alberta Terzi, Luca Fusaro, Davide Altamura, Francesca Boccafoschi, Oliver Bunk, Cinzia Giannini
Time scale of glycation in collagen of bovine pericardium-derived bio-tissues.
IuCrJ 8 (2021).
https://doi.org/10.1107/S2052252521010344
Cinzia Giannini, Liberato De Caro L, Alberta Terzi, Luca Fusaro, Davide Altamura, Ana Diaz, Rocco Lassandro, Francesca Boccafoschi, Oliver Bunk
Decellularized pericardium tissues at increasing glucose, galactose and ribose concentrations and at different time points studied using scanning X-ray microscopy.
IuCrJ 8, 621 (2021).
https://doi.org/10.1107/S2052252521005054
Cinzia Giannini, Alberta Terzi, Luca Fusaro, Teresa Sibillano, Ana Diaz, Martina Ramella, Viviane Lutz-Bueno, Francesca Boccafoschi, Oliver Bunk
Scanning X‐ray microdiffraction of decellularized pericardium tissue at increasing glucose concentration.
Journal of Biophotonics 12, e201900106 (2019).
https://doi.org/10.1002/jbio.201900106