Project details
This project aims at investigating and optimizing of a particular class of carbon-based materials for energy applications: fullerene and metal fullerides. In particular, we aim at improving their reactivity to hydrogen in order to improve their solid-state hydrogen storage capacity, which is to be achieved by heteroatom substitution and light metal coverage of the basically fullerene- and graphite-like structures.
It is well known that graphite and fullerene in pure forms cannot store appreciable amount of hydrogen, as required for technological applications. Intercalated graphite compounds, formed by insertion of alkali and alkaline earth metals into the interlayer or intermolecular regions, can however represent the substrate materials to store hydrogen. In terms of this project, we search for high-performance materials for hydrogen storage and aim at going beyond the state-of-art for the known carbon-based materials and investigate new possible candidate compounds.
Some of the recent results within this area of our research are:
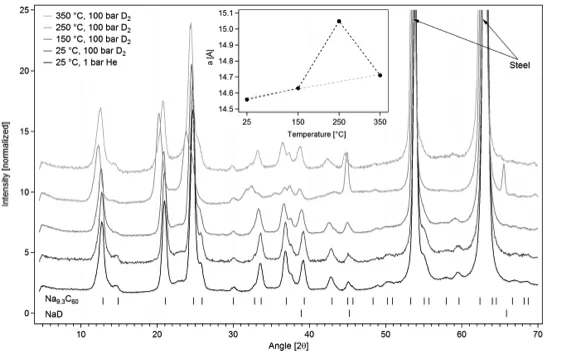
Study of the reversible hydrogen absorption in sodium intercalated fullerenes [1]
Hydrogen absorption of sodium-intercalated fullerenes NaxC60 was determined and compared to pure fullerenes C60. Up to 3.5 mass% hydrogen can reversibly be absorbed in NaxC60 (x~8…10) at 200°C and a hydrogen pressure of 200 bar, and the onset of hydrogen absorption is observed already at 150°C. At a pressure of 1 bar hydrogen the major desorption starts at 250°C and is completed at 300°C. This absorption and desorption temperatures are significantly reduced compared to pure C60. The compounds were investigated by a combination of X-ray, in-situ neutron powder diffraction and infrared spectroscopy, as well as by extensive hydrogen uptake/release studies. By using neutron diffraction we were able to show that the hydrogen ab/desorption is accompanied by a partial de/reintercalation of sodium. A minor part of the hydrogen is ionically bonded in NaH and the major part is covalently bonded in C60Hx. The sample can be fully dehydrogenated and no NaH is left after desorption. In contrast to C60, where the fullerene cages for high hydrogen loadings are destroyed during the sorption process, the NaxC60 sample stays intact.
Study of the reversible hydrogen absorption in sodium intercalated fullerenes [1]
Hydrogen absorption of sodium-intercalated fullerenes NaxC60 was determined and compared to pure fullerenes C60. Up to 3.5 mass% hydrogen can reversibly be absorbed in NaxC60 (x~8…10) at 200°C and a hydrogen pressure of 200 bar, and the onset of hydrogen absorption is observed already at 150°C. At a pressure of 1 bar hydrogen the major desorption starts at 250°C and is completed at 300°C. This absorption and desorption temperatures are significantly reduced compared to pure C60. The compounds were investigated by a combination of X-ray, in-situ neutron powder diffraction and infrared spectroscopy, as well as by extensive hydrogen uptake/release studies. By using neutron diffraction we were able to show that the hydrogen ab/desorption is accompanied by a partial de/reintercalation of sodium. A minor part of the hydrogen is ionically bonded in NaH and the major part is covalently bonded in C60Hx. The sample can be fully dehydrogenated and no NaH is left after desorption. In contrast to C60, where the fullerene cages for high hydrogen loadings are destroyed during the sorption process, the NaxC60 sample stays intact.
Synthesis and investigations of the new intercalated fullerene polymer Mg2C60 [2]
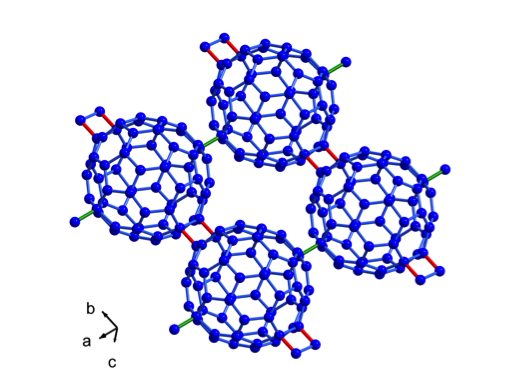
High resolution powder synchrotron and neutron diffraction data have clearly shown that Mg2C60 is isostructural to the superionic conductor Li4C60, where fullerenes form a two-dimensional network connected either by four-membered carbon rings, or single C–C bonds. Because of its peculiar structural arrangement, Mg2C60 behaves as a good ionic conductor by means of uncorrelated ionic hopping across very small energy barriers, thus suggesting its possible use in future Mg-ion batteries.
Fig. 2: The two-dimensional polymeric arrangement in Mg2C60, which is isostructural to Li4C60. The structure consists of a network of fullerene chains, linked by four-membered rings, interconnected by single C-C bonds running along orthogonal directions. The C60 units are significantly elongated due to the presence of inter-fullerene covalent bonds.
Collaborations
- Philippe Mauron, Arndt Remhof, Andreas Bliersbach, Andreas Borgschulte, Andreas Züttel, Empa. Swiss Federal Laboratories for Materials Science and Technology, Division "Hydrogen and Energy", Überlandstrasse 129, 8600 Dübendorf, Switzerland
- Daniele Pontiroli, Mattia Gaboardi, Marcello Mazzani, Mohammad Choucair, Matteo Aramini, Alessandra Gorreri, Mauro Riccò, Dipartimento di Fisica, Universita` di Parma, Via G. Usberti 7/a, 43124 Parma, Italy
References
- [1] P. Mauron, A. Remhof, A. Bliersbach, A. Borgschulte, A. Zuettel, D. Sheptyakov, M. Gaboardi, M. Choucair, D. Pontiroli, M. Aramini, A. Gorreri, M. Ricco, “Reversible hydrogen absorption in sodium intercalated fullerenes”, International Journal of Hydrogen Energy 37, 14307 (2012).
- [2] Daniele Pontiroli, Matteo Aramini, Mattia Gaboardi, Marcello Mazzani, Alessandra Gorreri, Mauro Ricco, Irene Margiolaki, Denis Sheptyakov, “Ionic conductivity in the Mg intercalated fullerene polymer Mg2C60”, Carbon 51, 143-147 (2013).