Show filters
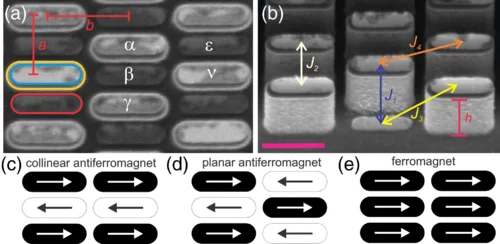
Geometrical Frustration and Planar Triangular Antiferromagnetism in Quasi-Three-Dimensional Artificial Spin Architecture
We present a realization of highly frustrated planar triangular antiferromagnetism achieved in a quasi-three-dimensional artificial spin system consisting of monodomain Ising-type nanomagnets lithographically arranged onto a deep-etched silicon substrate. We demonstrate how the three-dimensional spin architecture results in the first direct observation of long-range ordered planar triangular antiferromagnetism, in addition to a highly disordered phase with short-range correlations, once competing interactions are perfectly tuned. Our work demonstrates how escaping two-dimensional restrictions can lead to new types of magnetically frustrated metamaterials.
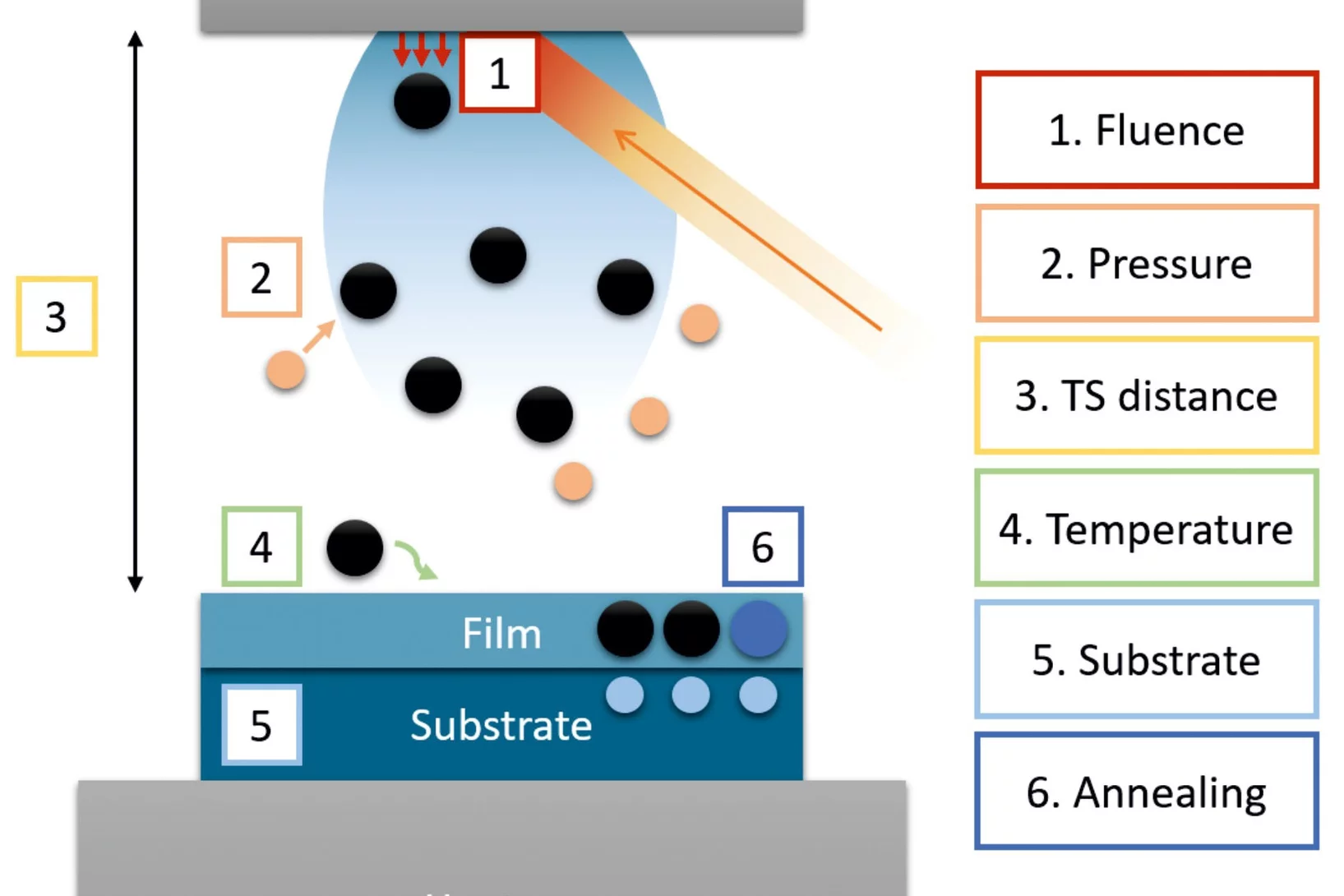
Pulsed Laser Deposition as a Tool for the Development of All Solid-State Microbatteries
All-solid-state lithium ion batteries (LIB) are currently the most promising technology for next generation electrochemical energy storage. Many efforts have been devoted in the past years to improve performance and safety of these devices. Nevertheless, issues regarding chemical and mechanical stability of the different components still hinder substantial improvements. Pulsed laser deposition (PLD) has proved to be an outstanding technique for the deposition of thin films of materials of interest for the fabrication of LIB. Thanks to its versatility and possible fine tuning of the thin film properties, PLD promises to be a very powerful tool for the fabrication of model systems which would allow to study in detail material properties and mechanisms contributing to LIB degradation. Nevertheless, PLD presents difficulties in the deposition of LIB components, mainly due to the presence of elements with large difference of atomic mass in their chemical composition. In this review, we report the main challenges and solution strategies used for the deposition through PLD of complex oxides thin films for LIB.
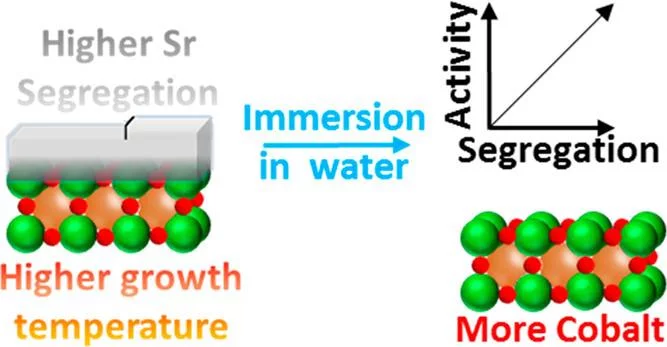
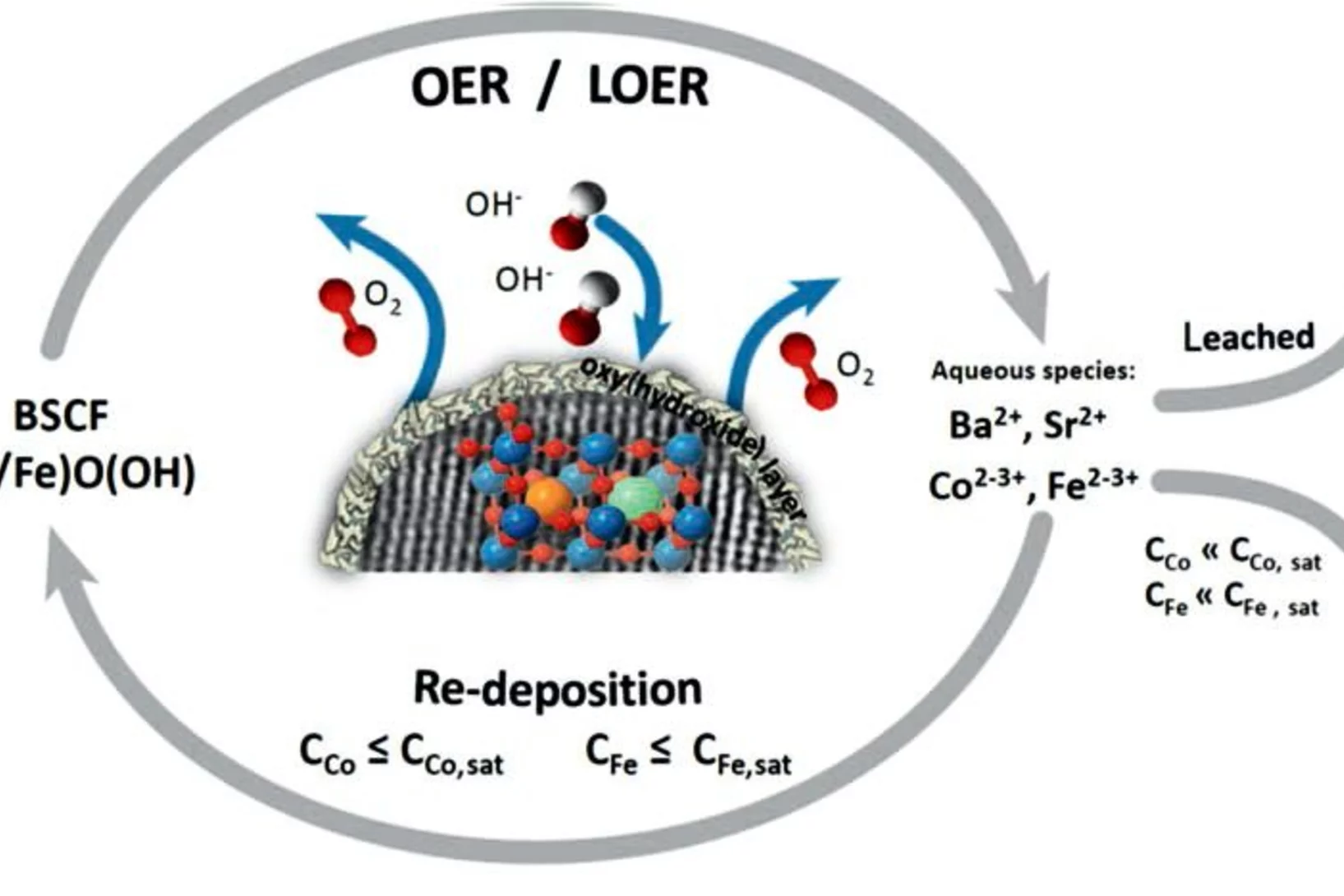
Surface Segregation Acts as Surface Engineering for the Oxygen Evolution Reaction on Perovskite Oxides in Alkaline Media
La1–xSrxCoO3-δ perovskites are potential catalysts for the anodic reaction of alkaline water electrolyzers, i.e., the oxygen evolution reaction (OER). It is well-known that La1–xSrxCoO3−δ perovskites can easily display strontium surface segregation, but how this influences the performance of La1–xSrxCoO3−δ perovskites as anodic electrode in alkaline water electrolyzers, particularly in terms of OER activity, has not been unveiled yet. This study focuses on La0.2Sr0.8CoO3−δ, which shows relatively high activity for the OER, and reveals the influence of the preparation temperature on the amount and morphology of segregated strontium-containing islands. Thin film samples were prepared at different temperatures by using pulsed laser deposition. Those samples were then characterized with synchrotron-based X-ray photoelectron spectroscopy “as prepared” and after being immersed in ultrapure water. We found that higher preparation temperatures enhance the segregation of strontium, which is then almost quantitatively removed by washing the samples with ultrapure water. After immersion in water, the samples expose a cobalt-rich surface. Investigating the OER activity as a function of the perovskite deposition temperature, it has been found that the higher the deposition temperature (i.e., the more extended the strontium segregation), the higher the OER activity. Such an effect has been linked to the higher amount of cobalt accessible after removing the strontium segregated islands.
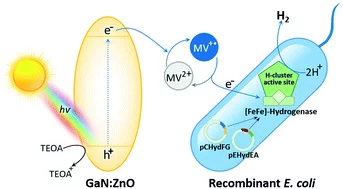
Photobiocatalytic H2 evolution of GaN:ZnO and [FeFe]-hydrogenase recombinant Escherichia coli
The need for sustainable, renewable and low-cost approaches is a driving force behind the development of solar-to-H2 conversion technologies. This study aims to develop a new strategy using a visible-light photocatalyst coupled to a biocatalyst for H2 production. Photocatalytic methyl viologen (MV2+) reduction activity was investigated to discover active oxynitrides. In comparative studies with LaTiO2N, BaTaO2N and Ta3N5, it was revealed that the suitable surface area, band gap and band edge potentials are some physical factors that are responsible for the photocatalytic behaviors of GaN:ZnO in MV2+ reduction. The activity is enhanced at higher concentrations and the alkaline pH of triethanolamine (TEOA). The expression of an active [FeFe]-hydrogenase from Escherichia coli (Hyd+E. coli) as a recombinant biocatalyst was confirmed by its MV˙+-dependent H2 production activity. In the photobiocatalytic system of GaN:ZnO and Hyd+E. coli, the rate of H2 production reached the maximum level in the presence of MV2+ as an electron mediator at neutral pH as a biocompatible condition. The present work reveals a novel hybrid system for H2 production using visible-light active GaN:ZnO coupled to Hyd+E. coli, which shows the feasibility of being developed for photobiocatalytic H2 evolution under solar light.
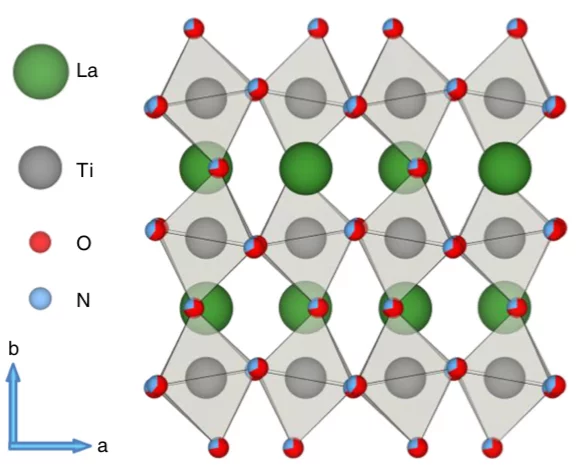
Examining the surface evolution of LaTiOxNy an oxynitride solar water splitting photocatalyst
LaTiOxNy oxynitride thin films are employed to study the surface modifications at the solid- liquid interface that occur during photoelectrocatalytic water splitting. Neutron reflectometry and grazing incidence x-ray absorption spectroscopy were utilised to distinguish between the surface and bulk signals, with a surface sensitivity of 3 nm.
Energy Conversion Processes with Perovskite-type Materials
Mixed oxides derived from the perovskite structure by combination of A- and B-site elements and by partial substitution of oxygen provide an immense playground of physico-chemical properties. Here, we give an account of our own research conducted at the Paul Scherrer Institute on perovskite-type oxides and oxynitrides used in electrochemical, photo(electro)chemical and catalytic processes aimed at facing energy relevant issues.
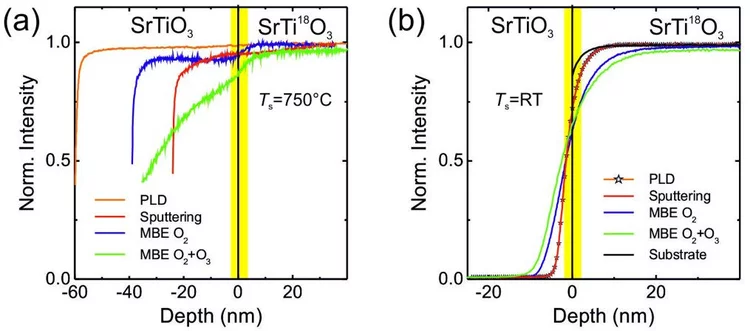
Oxygen diffusion in oxide thin films grown on SrTiO3
SrTiO3 thin films were grown on 18O-exchanged SrTiO3 single crystalline substrates by pulsed-laser deposition, rf sputtering, and oxide molecular-beam epitaxy to study their oxygen diffusion depth profiles using secondary ion mass spectrometry and elastic recoil detection analysis depth profiling. The oxygen depth profiling shows that SrTiO3 films prepared with the three different deposition techniques will take oxygen from the substrate, even at room temperature. This confirms that the substrate is one possible oxygen source for the growth of oxide thin films independent of the physical vapor deposition technique employed. It was also found that a reactive oxygen environment changes the oxygen composition of the substrate during the growth of a film and partly replaces 18O with 16O up to a depth of several tens of nm. These findings imply that SrTiO3 and therefore other ion conducting oxide substrates, which are commonly used as platforms for thin film growth, can be considered capricious in nature with respect to oxygen chemistry and lattice constants.