Search
Damage-Repair Cycle in Hydrocarbon Based Membranes for Fuel Cells
The development of next generation fuel cell membranes based on aromatic hydrocarbon chemistry calls for a new antioxidant strategy to tackle radical induced membrane degradation. Although damage by radicals cannot be prevented, the formed aromatic intermediates can be repaired by a suitable additive. Fuel cell experiments demonstrate that the approach is viable on the device level and that repair is a catalytic mechanism.
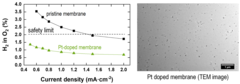
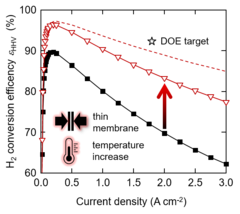
Enabling the use of Thin Membranes in Water Electrolyzers using a Recombination Catalyst
The conversion efficiency for green hydrogen production in a polymer electrolyte water electrolyzer (PEWE) is strongly influenced by the ohmic cell resistance and therefore the thickness of the membrane used. The use of thin membranes (~50 micron or below) is limited by gas crossover of H2 and O2, which can lead to the formation of explosive gas mixtures. The incorporation of a recombination catalyst provides remedy and allows a more dynamic operating mode.
Oxygen Evolution Reaction Activity and Underlying Mechanism of Perovskite Electrocatalysts at Different pH
PSI researchers have studied the how the electrolyte pH values influence the oxygen evolution reaction (OER) activity and stability of different promising perovskite oxide catalysts for application as anodic electrodes in alkaline water electrolyzers. The OER activity and stability decreased decreasing the electrolyte pH values. By combining electrochemical studies and operando X-ray absorption spectroscopy measurements, it has been suggested that different reaction mechanisms dominate in alkaline and near-neutral electrolyte pH region.
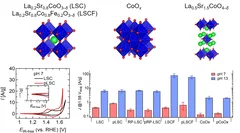
Surface segregation acts as surface engineering for the oxygen evolution reaction on perovskite oxides in alkaline media
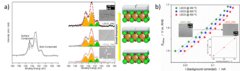
PSI researchers have studied the influence of surface segregation on the oxygen evolution reaction (OER) activity for the, La0.2Sr0.8CoO3-d (LSCO) perovskite, one of the most active perovskite towards the OER in alkaline electrolyte. It has been found that the higher the perovskite synthesis temperature the more strontium segregation occurs on the surface. However, the segregated strontium compounds are soluble in water and they are easily removed when the surface of the electrode is in contact with the electrolyte, leading to the exposure of cobalt enriched layers very active for the OER.
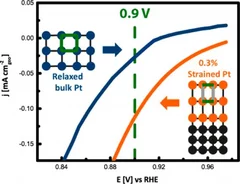
Investigating the Role of Strain toward the Oxygen Reduction Activity on Model Thin Film Pt Catalysts
Environmentally friendly energy conversion devices such as fuel cells are becoming more and more attractive. However, major impediments to large-scale application still arise on the material side, related to the cost and poor performance of the cathode catalyst. State-of-the-art electrocatalysts are all Pt-based materials, suffering from poor electrochemical oxygen reduction kinetics.
First direct observation of the oxygen transport in polymer electrolyte water electrolysis
PSI researchers have developed a new methodology for studying the complex transport processes in polymer electrolyte water electrolysis (PEWE). Using advanced operando X-ray tomographic microscopy, we were able to observe for the first time the formation of oxygen pathways in the porous transport layer, in three dimensions. Understanding oxygen transport is crucial for improving PEWE technology and this work provides precious insights for the design of future, better-performing PEWE cells.
Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
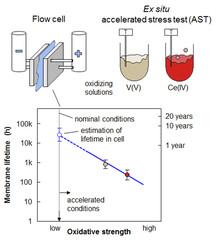
Membrane Lifetime Estimation in a Vanadium Redox Flow Battery using an Accelerated Stress Test
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
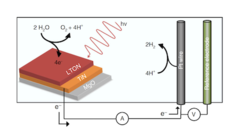
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
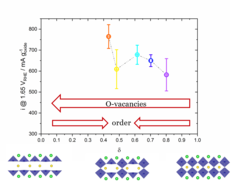
Correlation between Oxygen Vacancies and Oxygen Evolution Reaction Activity for a Model Electrode: PrBaCo2O5+δ
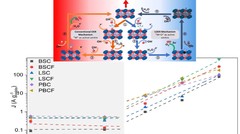
The role of the oxygen stoichiometry of perovskite catalysts in the oxygen evolution reaction (OER) is systematically studied in the PrBaCo2O5+δ family. The reduced number of physical/chemical variables combined with in-depth characterizations such as neutron diffraction, O K-edge X-ray absorption spectroscopy(XAS), electron energy loss spectroscopy (EELS), magnetization and scanning transmission electron microscopy (STEM) studies, helps investigating the complex correlation between OER activity and a single perovskite property, such as the oxygen content. Larger amount of oxygen vacancies appears to facilitate the OER, possibly contributing to the mechanism involving the oxidation of lattice oxygen, i.e., the lattice oxygen evolution reaction (LOER). Furthermore, not only the number of vacancies but also their local arrangement in the perovskite lattice influences the OER activity, with a clear drop for the more stable, ordered stoichiometry.
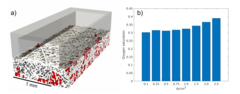
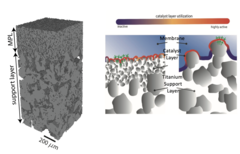
Hierarchically Structured Porous Transport Layers for Polymer Electrolyte Water Electrolysis
The high operational and capital costs of polymer electrolyte water electrolysis technology originate from limited catalyst utilization and the use of thick membrane electrolytes. PSI researchers have developed novel multi-layer porous transport materials, which provide superior electrochemical performance in comparison to conventional single-layer structures.
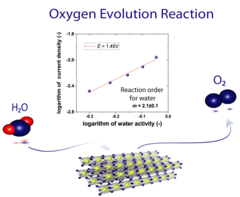
Understanding of the Oxygen Evolution Reaction Kinetics in Acidic Environment
The high operational expenditure of polymer electrolyte water electrolysis (PEWE) technology, dominated by kinetic losses from the sluggish oxygen evolution reaction (OER), inhibits large-scale market penetration. PSI researchers have developed a novel methodology to access underlying reaction mechanism of the OER. For the first time the reaction order for water has been determined. Advanced benchmarking of catalysts in technical environment also supports the development of novel, highly efficient catalyst materials.
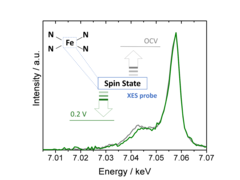
Using X-ray emission spectroscopy to study the electronic properties of single atom catalysts
Single atom catalysts hold great promise as O2- or CO2-reduction electrocatalysts, but a deeper understanding of their active sites’ structure and electronic properties is needed in order to render them sufficiently active and stable. To this end, we have used X-ray emission spectroscopy to determine these catalysts’ electronic configuration, and performed in situ measurements that unveil the effect of potential on this key feature.
Efficient Water Electrolysis at Elevated Temperature using Commercial Cell Components
Decarbonization of the energy system across different sectors using power-to-X concepts relies heavily on the availability of low-cost hydrogen produced from renewable power by water electrolysis. Polymer electrolyte water electrolysis (PEWE) is a promising technology for hydrogen (and oxygen) production for distributed as a well as centralized operation. The total cost of hydrogen is dominated by the electricity cost. Therefore, increase of conversion efficiency is pivotal in improving the commercial viability of electrolytically produced hydrogen. In this study, we investigate the prospects of improving conversion efficiency by reducing the membrane thickness from 200 to 50 micron and increasing the cell temperature from 60 to 120°C.