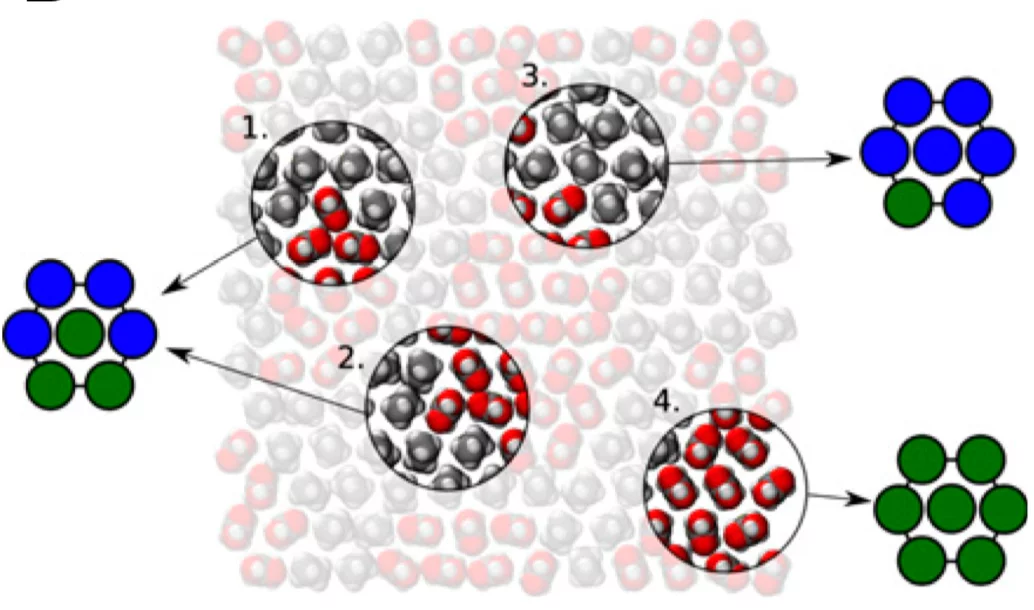
Every folded protein presents an interface with water that is composed of domains of varying hydrophilicity/-phobicity. Many simulation studies have highlighted the nonadditivity in the wetting of such nanostructured surfaces in contrast with the accepted theoretical formula that is additive. We present here an experimental study on surfaces of identical composition but different organization of hydrophobic and hydrophilic domains. We prove that the interfacial energy of such surfaces differs by ∼20% and that a significant difference in the interfacial water H- bonding structure can be measured. As a result, in combination with molecular-dynamics simulations, we propose a model that captures the wetting of molecularly heterogeneous surfaces, showing the importance of local structure (first-nearest neigh- bors) in determining the wetting properties.
Facility: SINQ
Reference: Z. Luo et al, PNAS 116, 25516 (2019)
Read full article: here