Project motivation
Unraveling the synthesis mechanism of solid compounds is of enormous interest for both academia and industry due to potential possibility to engineer synthesis of materials with specific properties for target applications. Needless to say, studying the crystallization processes is complex and requires physical chemical methods, which can monitor the evolution of the species in both liquid and solid phase. The synthesis of solid materials is a complex process, because of the heterogeneity of the synthesis medium, which can comprise solvent, inorganic precursors, organic linkers, modulators, templates, etc. Moreover, it depends on various physical and chemical parameters, such as temperature, pressure, pH, composition of the reaction mixture, nature of the solvent, and ageing time.
In situ time-resolved spectroscopy
To obtain complete and, most importantly, reliable information about the processes occurring during the synthesis, techniques that allow direct observation (in situ) are required. However, in situ investigation of especially solvothermal synthesis is associated with significant experimental difficulties related to the severe synthesis conditions required (temperature up to 200 °С, pressure up to 20 bar, organic solvents, acidic or alkali media) and the complexity of the systems, which usually comprises various phases: true solutions, colloid solutions, precipitates, nanosized crystalline intermediates and the final product. In this respect, studying the synthesis of the high-performance materials, such as zeolites and metal-organic frameworks, faces significant obstacles.
Custom in situ cells
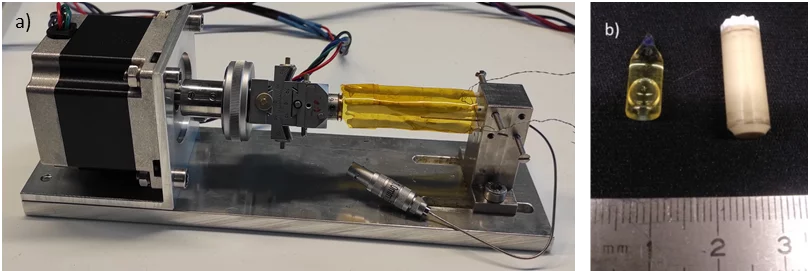
To overcome these problems, we design and develop custom in situ cells, which are able to work under severe conditions, enabling acquisition of the time-resolved data. To acquire time-resolved in situ XAS spectra and X-ray scattering data, the custom cell enabling heating and spinning of the capillary with the synthetic mixture is available. It can be installed at beamline or lab-based XAS spectrometer or diffractometer and be used in transmission, fluorescence or scattering mode. For collecting NMR spectra, standard probes, which can accommodate 4 and 7 mm rotors spinning under magic angle for the detection of both liquid phase and solid species, are utilized. A glass insert which can be tightly placed in zirconia rotor are filled with the synthetic mixture used for the preparation of studied material. Commercial NMR probes and XAS cell both allow continuous in situ heating up to 130 °C, and with some upgrade till 200 °C which is sufficient for studying the synthesis of the majority of the functional materials.
Materials in the focus
Metal-organic frameworks
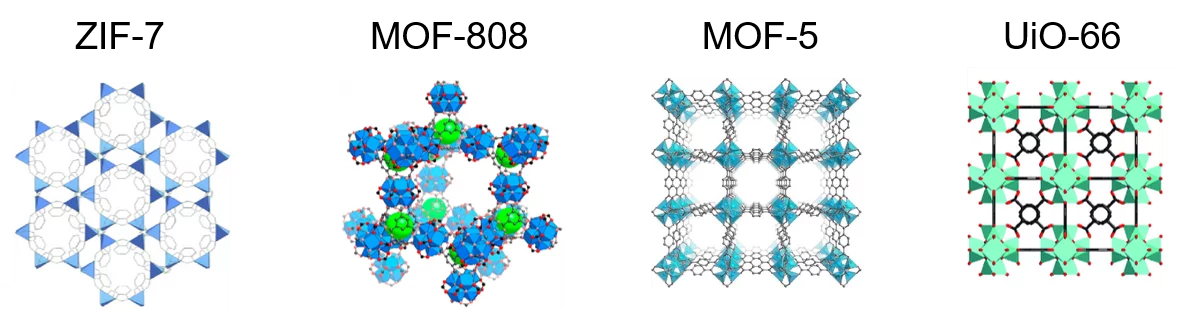
Metal-organic frameworks (MOFs) attract great attention by both academia and industry due to their unique properties, including very large surface area, variable size of the pores, possibility to tune the nature and location of functional groups and defects, which make them effective adsorbents and catalysts. Therefore, MOFs are used in many fields, including gas storage, gas separation, heterogeneous catalysis, electrochemical fuel cells, and batteries. Notwithstanding these significant achievements, further progress in the application of MOFs is hindered by a poor understanding and the lack of knowledge on the mechanisms of their formation. MOF synthesis relies mostly on screening of many compositions and synthesis conditions and is based on trial and error. Knowing the mechanism of the synthesis in turn enables tailoring the final properties of MOFs, such as structural and textural composition, crystallinity, nature and number of defects, acidity, and redox properties. The rational design of materials with their desired properties requires a deeper insight in the mechanisms of formation of MOFs, which is the ultimate goal of this project.
Zeolites and molecular sieves
Zeolites are among the most important heterogeneous catalysts. Nanomaterials based on micro- and mesoporous sieves represent a unique class of compounds with the ordered structure forming 3D system of uniform molecule-size pores. Due to peculiarities of pore structure and geometry, these materials can selectively adsorb molecules with the corresponding size, and therefore they can evince properties of molecular sieves. Along with molecular sieve properties zeolite, aluminophosphate, mesoporous and micro-mesoporous sieves show acid-base and redox properties and display high catalytic activity in the number of heterogeneous catalytic processes. Owing to these properties inorganic molecular sieves are widely applied refinery, gas and oil conversion, organic and fine organic syntheses. More than 80 heterogeneous catalytic processes are based on molecular sieve catalysts in the modern fuel and energy complex of the world’s developed countries. However, the mechanism of active site formation in the course of synthesis of molecular sieve catalysts remains unclear, since the techniques of the direct monitoring of the processes occurring during the synthesis of these materials are lacking.