Search
Electrocatalysis and Interfaces
The Electrocatalysis and Interfaces Group was established in 2012 combining the electrocatalysis activites of the former Fuel Cell Group and the Interface analytical activities of the former Interface and Capacitor Group. Electrocatalysis is the key topic for electrochemical energy conversion.
Damage-Repair Cycle in Hydrocarbon Based Membranes for Fuel Cells
The development of next generation fuel cell membranes based on aromatic hydrocarbon chemistry calls for a new antioxidant strategy to tackle radical induced membrane degradation. Although damage by radicals cannot be prevented, the formed aromatic intermediates can be repaired by a suitable additive. Fuel cell experiments demonstrate that the approach is viable on the device level and that repair is a catalytic mechanism.
Publications 2015
Electrochemistry Laboratory (LEC)
Publications 2018
Electrochemistry Laboratory (LEC)
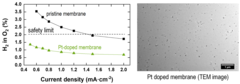
Enabling the use of Thin Membranes in Water Electrolyzers using a Recombination Catalyst
The conversion efficiency for green hydrogen production in a polymer electrolyte water electrolyzer (PEWE) is strongly influenced by the ohmic cell resistance and therefore the thickness of the membrane used. The use of thin membranes (~50 micron or below) is limited by gas crossover of H2 and O2, which can lead to the formation of explosive gas mixtures. The incorporation of a recombination catalyst provides remedy and allows a more dynamic operating mode.
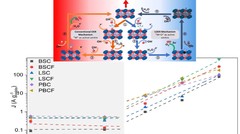
Oxygen Evolution Reaction Activity and Underlying Mechanism of Perovskite Electrocatalysts at Different pH
PSI researchers have studied the how the electrolyte pH values influence the oxygen evolution reaction (OER) activity and stability of different promising perovskite oxide catalysts for application as anodic electrodes in alkaline water electrolyzers. The OER activity and stability decreased decreasing the electrolyte pH values. By combining electrochemical studies and operando X-ray absorption spectroscopy measurements, it has been suggested that different reaction mechanisms dominate in alkaline and near-neutral electrolyte pH region.
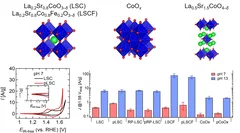
Surface segregation acts as surface engineering for the oxygen evolution reaction on perovskite oxides in alkaline media
PSI researchers have studied the influence of surface segregation on the oxygen evolution reaction (OER) activity for the, La0.2Sr0.8CoO3-d (LSCO) perovskite, one of the most active perovskite towards the OER in alkaline electrolyte. It has been found that the higher the perovskite synthesis temperature the more strontium segregation occurs on the surface. However, the segregated strontium compounds are soluble in water and they are easily removed when the surface of the electrode is in contact with the electrolyte, leading to the exposure of cobalt enriched layers very active for the OER.
Publications 2009
Electrochemistry Laboratory (LEC)
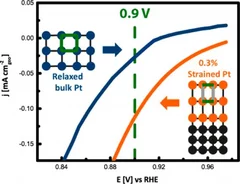
Investigating the Role of Strain toward the Oxygen Reduction Activity on Model Thin Film Pt Catalysts
Environmentally friendly energy conversion devices such as fuel cells are becoming more and more attractive. However, major impediments to large-scale application still arise on the material side, related to the cost and poor performance of the cathode catalyst. State-of-the-art electrocatalysts are all Pt-based materials, suffering from poor electrochemical oxygen reduction kinetics.
Publications 2012
Electrochemistry Laboratory (LEC)
First direct observation of the oxygen transport in polymer electrolyte water electrolysis
PSI researchers have developed a new methodology for studying the complex transport processes in polymer electrolyte water electrolysis (PEWE). Using advanced operando X-ray tomographic microscopy, we were able to observe for the first time the formation of oxygen pathways in the porous transport layer, in three dimensions. Understanding oxygen transport is crucial for improving PEWE technology and this work provides precious insights for the design of future, better-performing PEWE cells.
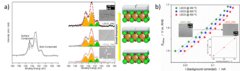
Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
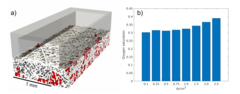
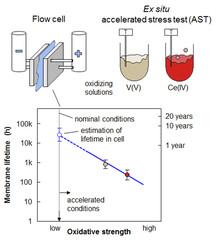
Membrane Lifetime Estimation in a Vanadium Redox Flow Battery using an Accelerated Stress Test
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
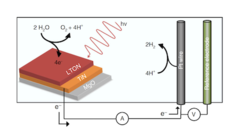
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.