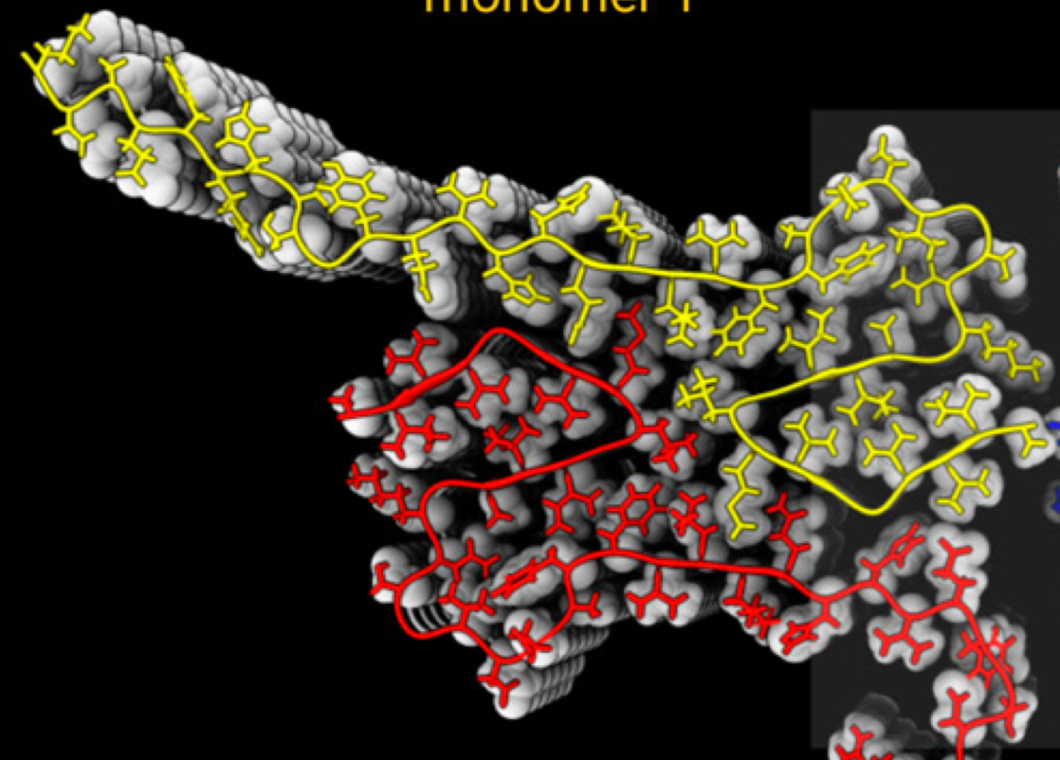
Amyloid β 42 fibril structure based on small-angle scattering
Alzheimers disease is one of the major global health challenges. Neuronal cell dysfunction and death are connected to the self-assembly of the amyloid β peptide (Aβ42) into oligomeric and fibrillar aggregates. The fibril surface can catalyze the formation of toxic oligomers via secondary nucleation. Access to a high-resolution structure of Aβ42 fibrils would provide a valuable basis for design of inhibitors of oligomer generation and toxicity in the form of fibril-binders and thus significantly contribute to the development of therapeutics against Alzheimer’s disease. A combination of methods may be most fruitful toward this aim. We show that small-angle X-ray scattering data, in combination with a solid-state NMR structure of the filament core, can reveal a detailed fibril model.