News & Scientific Highlights
HERCULES SCHOOL 2021 AT PSI
During the week of March 15 – 19, we had the pleasure to welcome 20 international PhD students, PostDocs and assistant professors at PSI, taking part in the first virtual HERCULES SCHOOL on Neutrons & Synchrotron Radiation.
Ruzicka Prize
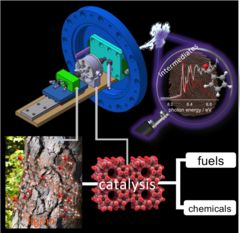
The Ružička Prize 2020 goes to Dr. Patrick Hemberger (PSI) for his research on understanding the mechanisms of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysts.
Methylbismuth: First observation of an organometallic biradical reactive intermediate
Open shell organometallic bismuth are promising agents for catalytic applications, but difficult to characterize due to their high reactivity. The simplest methylbismuth (Bi-CH3), a biradical species, was in-situ synthesized and spectroscopically characterized for the first time. Electronic and thermochemical properties could be obtained, which will guide future synthetic applications.
Selective Alkane Functionalization to Olefins
Light alkanes are abundantly available and cheap resources that are often burned at oil wells because of the missing infrastructure for valorization. Novel technologies are needed for their selective functionalization to use natural gas as an energy vector in the transition between the oil and the renewables era. Catalytic oxyhalogenation may unlock the transformation of cheap and abundant alkanes into commodities. When chlorine-based reactions are compared with bromine, improved selectivities above an iron catalyst arise from surface-confinement of the reaction mechanism in the case of chlorine as halogen.
How the methyl group position influences the ultrafast deactivation in aromatic radicals
The resonantly stabilized xylyl radicals (C8H9•) distinctively influence the combustion chemistry and, therefore, ultimately determine the performance of combustion engines. At that, the three different isomers (methyl group in ortho, para or meta position) exhibit notable differences at elevated temperatures. We have tracked down these dynamics on a femtosecond timescale by monitoring the response to preparation of a well-defined electronic and vibrational state.
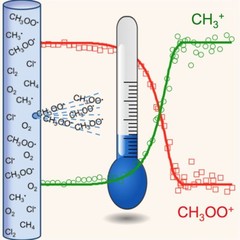
Radical Thermometers and Energetics
Methylperoxy radicals are crucial oxidation intermediates and could be synthesized photolytically in an exothermic reaction. Despite their vanishingly small concentration, their temperature could be measured after a few ten thousand collisions inside the reactor, which opens up the possibility of time-resolved operando temperature measurements. Also the reaction energy to yield methyl cations and oxygen could be determined with sub-kJ mol–1 precision, which firmly anchors the methylperoxy energetics to that of well-known stable species and opens up the possibility of highly accurate radical thermochemistry measurements.
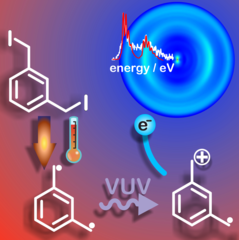
Taming Reactive Molecular Magnets
Studying organic molecular magnets is a challenge, because the high-spin diradical character of these compounds dramatically increases the reactivity and reduces the lifetime. Researchers from PSI, ETH Zurich, Wollongong and Melbourne, Australia succeeded in taming the meta-xylylene diradical and were able to study its electronic and thermochemical properties.
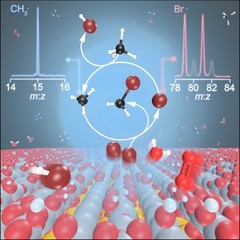
Tracking down radicals in methane oxybromination
Catalytic oxybromination may turn the cheap and abundant feedstock methane into the platform compounds bromomethane and dibromomethane. Yet researchers have been puzzled by the catalysis mechanism, which was speculated to involve free radical intermediates. Operando photoelectron photoion coincidence helped distinguish surface and gas-phase reaction steps and elucidated the crucial halogen-mediated C–H bond activation step, which is driven by elusive bromine and methyl radicals.
Understanding the reaction mechanism in lignin catalytic fast pyrolysis
Lignin is a major constituent of plants, and may be used as a precursor for fuels and fine chemicals. Catalytic fast pyrolysis of lignin is one of the most promising approaches. By using vacuum ultraviolet synchrotron radiation and threshold photoelectron spectroscopy we could identify elusive intermediates, which are responsible for the formation of phenol and benzene and could thus tackle this reaction mechanism. Mechanistic understanding could enable targeted improvement of production methods in the future, beyond the currently used "cook-and-look" approach.