Search
How important is hydrogen for the energy transition?
Assessments by PSI energy expert Thomas J. Schmidt
Energie et climat
Aperçu de sujets
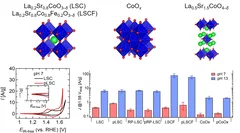
Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
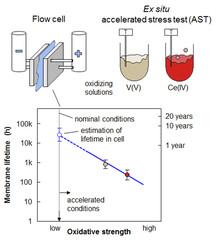
Membrane Lifetime Estimation in a Vanadium Redox Flow Battery using an Accelerated Stress Test
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
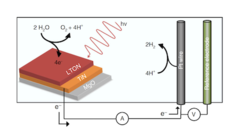
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
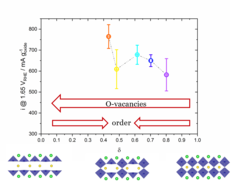
Correlation between Oxygen Vacancies and Oxygen Evolution Reaction Activity for a Model Electrode: PrBaCo2O5+δ
The role of the oxygen stoichiometry of perovskite catalysts in the oxygen evolution reaction (OER) is systematically studied in the PrBaCo2O5+δ family. The reduced number of physical/chemical variables combined with in-depth characterizations such as neutron diffraction, O K-edge X-ray absorption spectroscopy(XAS), electron energy loss spectroscopy (EELS), magnetization and scanning transmission electron microscopy (STEM) studies, helps investigating the complex correlation between OER activity and a single perovskite property, such as the oxygen content. Larger amount of oxygen vacancies appears to facilitate the OER, possibly contributing to the mechanism involving the oxidation of lattice oxygen, i.e., the lattice oxygen evolution reaction (LOER). Furthermore, not only the number of vacancies but also their local arrangement in the perovskite lattice influences the OER activity, with a clear drop for the more stable, ordered stoichiometry.