This project is dedicated to the study of complex ordering phenomena and associated changes in the physical and chemical properties in non-stoichiometric solid oxides by controlling the oxygen content in the oxide family RE2MO4+δ and related compounds. These compounds are complex due to the many active degrees of freedom such as charge, spin and orbital ordering, which interact in a competitive, synergetic way. Due to the many degrees of freedom and their complex interplay there is a high potential for tailoring new dedicated materials for various applications by optimising parameters through chemical doping. Therefore, these compounds are of great interest for applications in oxygen sensors, oxygen membranes or as electrolytes in solid-oxide fuel cells.
The complexity of these compounds is both a challenge and an opportunity. Their investigation requires growth of high-quality single crystals, the application of macroscopic and microscopic experimental methods, as well as a fundamental understanding of the physical and chemical processes. Consequently, this project includes the combination of complementary methods such as powder and single crystal diffraction by neutron and X-ray combined with maximum entropy and Rietveld data analysis. The RE2MO4+δ compounds display rich structural phase diagrams as a function of the excess oxygen content δ as well as the temperature, showing complex long-range ordering of excess oxygen atoms in oxygen over-stoichiometric phases, non-classical oxygen mobility in the room- and moderate-temperature regimes for some compositions, and exciting electronic and magnetic properties.
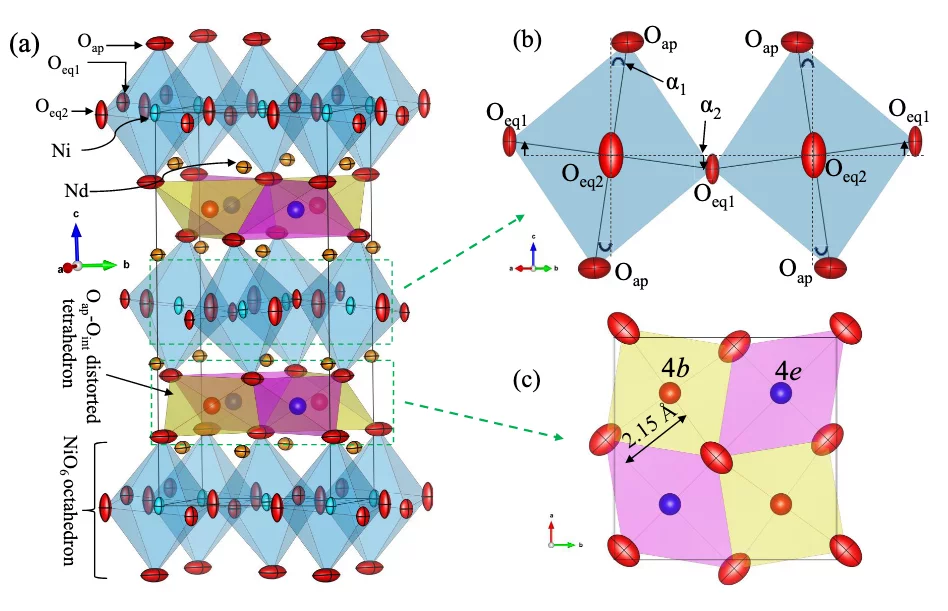
Recently, we have studied in detail the influence of oxygen overstoichiometry on apical oxygen disorder and magnetic correlations in Nd2NiO4+δ (δ ∼ 0.1) by means of synchrotron x-ray powder diffraction, neutron single-crystal and powder diffraction studies, combined with macroscopic magnetic measurements [1,2]. We found that oxygen diffusion along the [110] direction lead to distinctive local distortions of the respective tetrahedral sites, resembling a successively in- and exhaling behavior of the empty and occupied tetrahedra along the diffusion pathway. Moreover, strong anharmonic displacements of the apical oxygen atoms towards the nearest vacant interstitial sites are observed, induced by the presence of interstitial oxygen atoms. Such anharmonic behavior of the apical oxygens is interpreted to be an important prerequisite for a phonon-assisted oxygen diffusion mechanism close to room temperature.
Single crystal neutron diffraction on Nd2NiO4+δ (δ ∼ 0.1) revealed a remarkable interdependent scaling of long-range oxygen and magnetic order [1,3]. The magnetic order thus coexists below TN = 48 K with the 3D ordering of excess oxygen atoms in Nd2NiO4.10, which has not previously been encountered for the homologous nickelates. This clearly indicates the potential of the interstitial oxygen atoms to enable inter- and intralayer coupling for structural and magnetic ordering.
Publications
- S.R. Maity, "Interplay of chemical doping, crystal structure and magnetism in oxygen non-stoichiometric Nd2-xSrxNiO4+δ", doctoral thesis, University of Geneva, 2019
- S.R. Maity, M. Ceretti, L. Keller, J. Schefer, T. Shang, E. Pomjakushina, M. Meven, D. Sheptyakov, A. Cervellino, and W. Paulus, “Structural disorder and magnetic correlations driven by oxygen doping in Nd2NiO4+δ (δ ∼ 0.11)”, Phys. Rev. Materials 3, 083604 (2019)
- S.R. Maity, M. Ceretti, L. Keller, J. Schefer, M. Meven, E. Pomjakushina, and W. Paulus, “Interdependent scaling of long-range oxygen and magnetic ordering in nonstoichiometric Nd2NiO4.10”, Phys. Rev. Materials 5, 014401 (2021)
Collaboration
- Prof. Dr. W. Paulus, University of Montpellier, France
- Dr. M. Ceretti, University of Montpellier, France
- Dr. E. Pomjakushina, LMX/PSI
Funding
- SNSF Project No. 200021L_157131
Associated junior researcher
- Dr. S.R. Maity, PhD University of Geneva, 2019
Alumni
- Dr. J. Schefer, LNS/PSI