Development of nanoscale acoustic tweezers
Acoustic tweezers: a reusable sonic chip plus a disposable microfluidic cartridge that cleanly captures and gently squeezes living cells—featured on the August cover of IEEE T-UFFC.
Manipulating microscopic objects with sound—without waste or contamination.
Acoustic tweezers use sound waves to move and deform tiny objects, including living cells, without touching them. We’ve redesigned the platform so it’s both cleaner and more affordable: a reusable surface-acoustic-wave (SAW) chip mates to a disposable microfluidic cartridge, cutting per-experiment costs and minimizing cross-contamination.
What’s new
- By tuning the drive to coupled chip–channel resonances near 50 MHz, we generated standing waves delivering pressures up to ~2.1 MPa.
- Those fields captured and gently compressed fast-swimming Tetrahymena and human cells, enabling controlled, repeatable manipulation.
- Time-resolved imaging let us quantify how cells deform, opening label-free routes to probe cell mechanics.
Why it matters
Strong, well-controlled acoustic fields in a clean, swappable cartridge make it practical to study soft materials and biological samples with less hassle—and to compare results across days, users, and labs.
How we did it
- A hybrid SAW–silicon design concentrates acoustic energy into the channel.
- A standardized coupling and alignment scheme keeps pressure delivery consistent.
- Tracer-based calibration links drive settings to in-channel acoustic pressure.
What’s next
We’re building a rigid microscope holder for higher-power operation and integrating compact microfluidic pump/extractor modules for fully closed-loop control. On the assay side, we’re exploring multi-frequency microrheology and localized higher-frequency patches to probe subcellular mechanics.
Publication
Our study appears in IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control and is featured on the journal’s August cover.
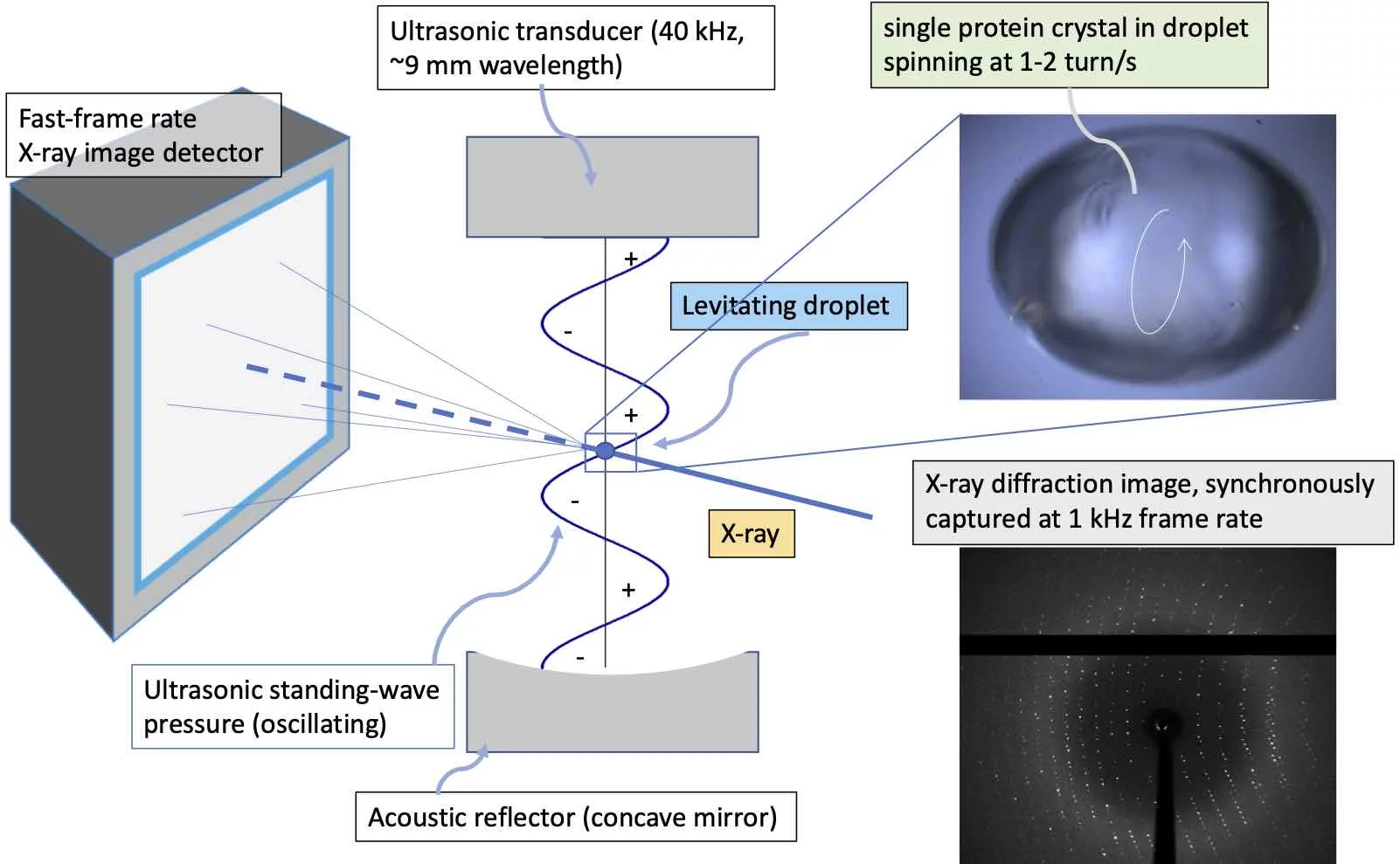
Development of Ultrasonic Acoustic Levitation Diffractometer
Ultrasonic acoustic levitation diffractometer (ALD) is a new tool for room-temperature protein crystallography that combines acoustic levitation, a fast pixelated X-ray detector, and a high-brightness X-ray beam. Our goal is a high-speed, sample-efficient, high-throughput workflow without mounting crystals on solid supports. The ALD is under development at the PXI (XSA06) protein crystallography beamline of the Swiss Light Source.
How it works
A highly brilliant X-ray microbeam irradiates a sample held in an acoustically levitated droplet. Acoustic streaming continuously rotates microcrystals within the droplet, while a fast, high-frame-rate detector records diffraction across orientations for structure solution at room temperature.
What we’ve demonstrated
- Fast solution mixing: an inertial-mixing method that accelerates exchange to ~0.1 s in sub-microliter to few-microliter droplets.
- Time-lapse crystallography: a pilot experiment capturing transient ligand binding using levitated droplets (see our article in Droplets).
Collaboration & support
This work is carried out with the Center for Photon Science and the Center for Scientific Computing, Theory and Data, and is supported by the Swiss National Science Foundation, Innosuisse (in collaboration with leadXpro AG), and the EU Horizon 2020 program.
Related publications & highlights
- “Ultrasonic acoustic levitation for fast-frame-rate X-ray protein crystallography at room temperature,” Scientific Reports (featured as a Scientific Highlight).
- “Acoustic levitation and rotation of thin films and their application for room-temperature protein crystallography,” Scientific Reports (Scientific Highlight; includes a demonstration video of easy film loading).
- “Inertial mixing of acoustically levitated droplets for time-lapse protein crystallography,” Droplets.
See also
- "Ultrasonic acoustic levitation for fast frame rate X-ray protein crystallography at room temperature" published in Scientific Reports and Scientific Highlight.
- "Acoustic levitation and rotation of thin films and their application for room temperature protein crystallography" published in Scientific Reports and Scientific Highlight with a video showing how easy to load these films onto an acoustic levitator.
Other projects by S. Tsujino: