- B.S. in Physics from the University of Maryland, College Park, USA, 2008.
- Ph.D. in Marine and Atmospheric Science from Stony Brook University (SBU), NY, USA, 2013.
- Postdoc in Interfacial photochemistry at the Institut de Recherches sur la Catalyse et l'Environnement de Lyon (IRCELYON) until 2016.
- Postdoc and currently Project Scientist at the Paul Scherrer Institute.
Research activities / Special interests
Environmental micro-reactor for atmospheric chemistry and physics
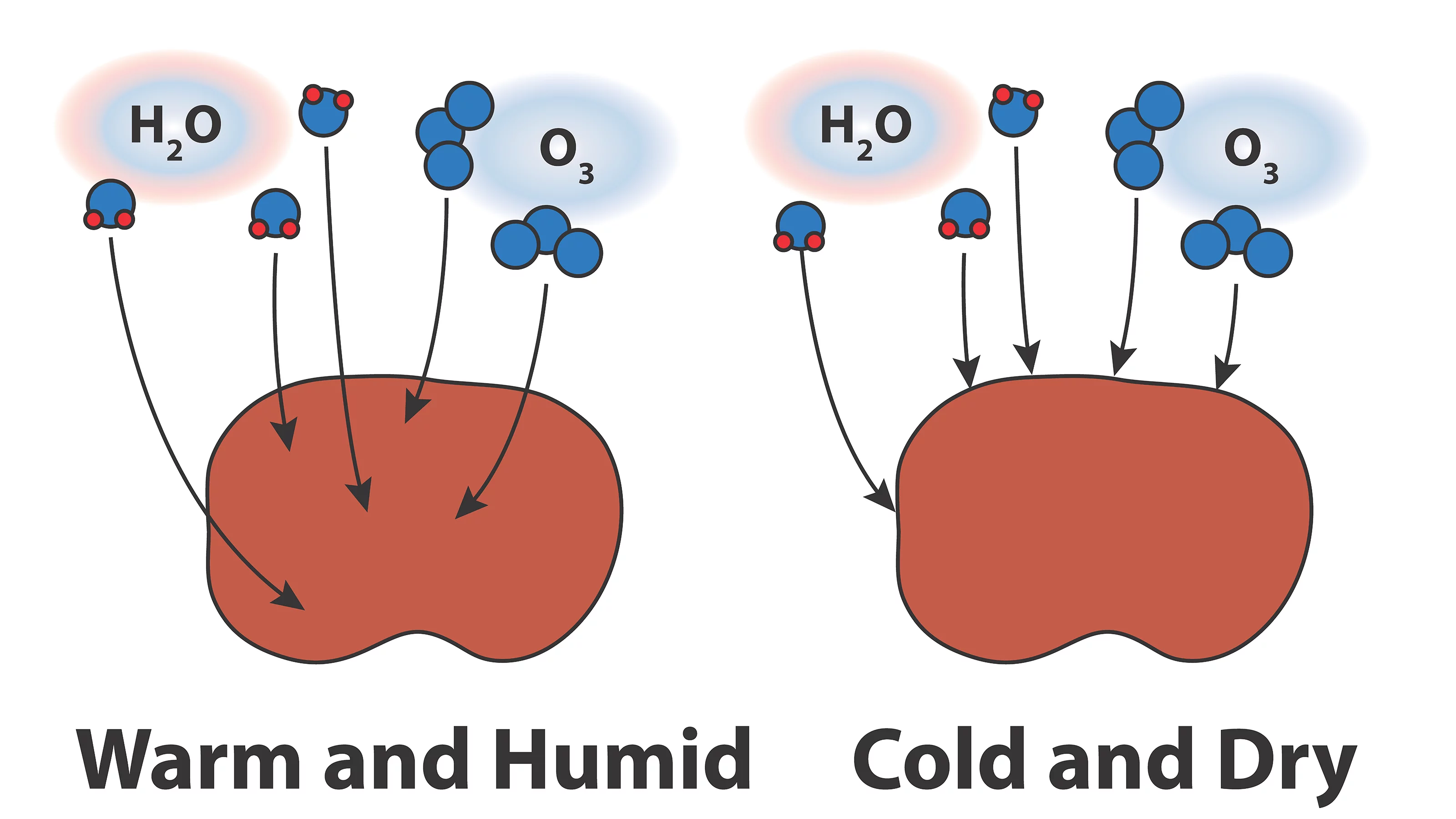
Organic particles can be highly viscous in air that is dry and cold. Therefore, it is important to quantify limitations of gases through organic materials as a function of temperature and relative humidity to better understand chemical and physical process involving atmospheric aerosol particles. Using state of the art spectro-microscopic techniques at the Swiss Light Source with the development of a new environmental micro-reactor, I investigate molecular diffusion of water vapor, ozone and other reactive gasses through organic particles less than 1 micrometer in diameter. At cold temperatures and low humidity, I expect that chemical reactions are limited to particle surfaces and microphysical processes such as ice nucleation and water condensation are altered.
Chemically mapping ice forming particles
https://www.psi.ch/en/luc/scientific-highlights/chemically-mapping-ice-…
Precipitation is mostly formed via the ice phase in mixed phase clouds and ice clouds are very relevant for Earths’ climate. Ice nucleation is the first step in freezing. Freezing or prevention of freezing is common to everyday life, e.g. for food and drug storage, icing and de-icing, etc. However, the ice nucleation process is not well understood, since it occurs on the size scale of clusters of molecules and time scales of molecular fluctuations.
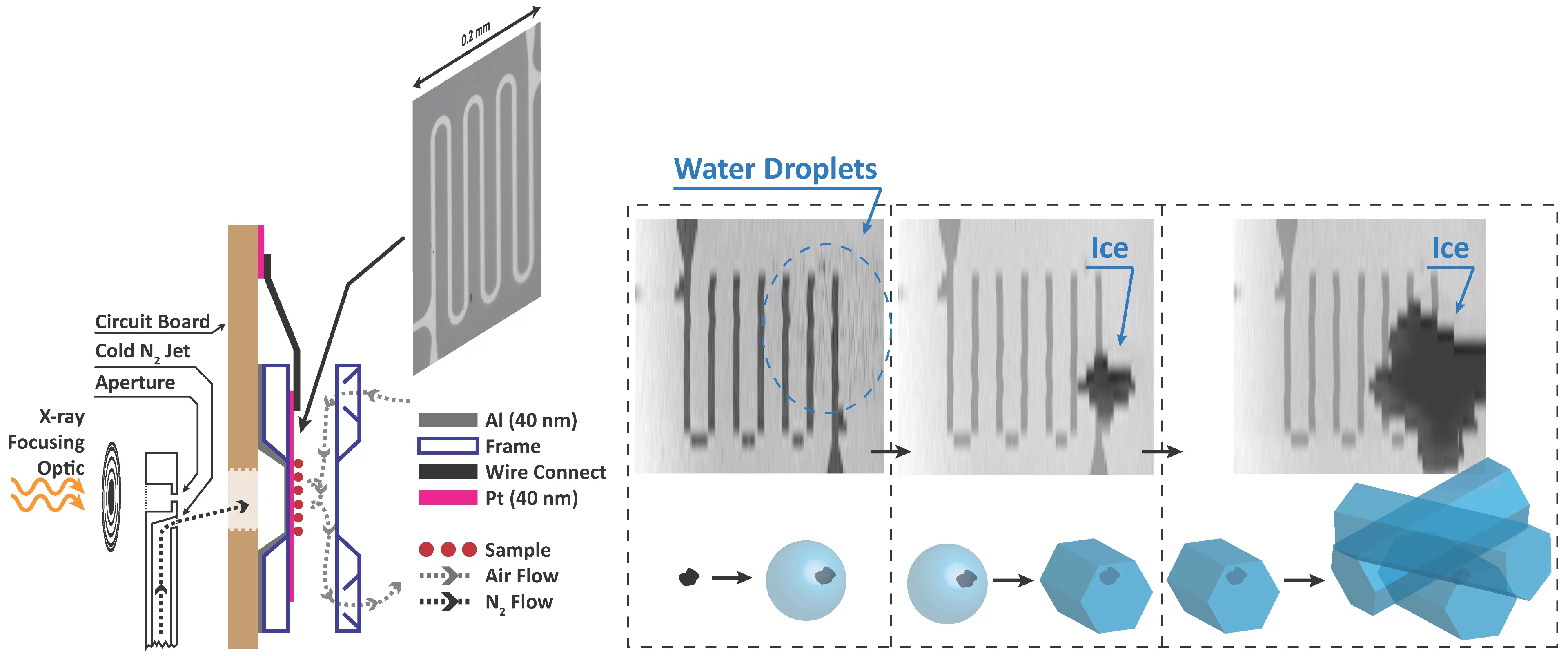
We built new instrumentation to observe when ice forms and use X-ray microscopy and spectroscopy to track the physical and chemical properties of ice nucleating particles with 35 nm spatial resolution. This is the first time ice nucleation has been measured in-situ in an X-ray microscope. The main technical challenge was temperature, and thus relative humidity control, while maintaining X-ray transparency. A sketch of our setup is shown. In a standard configuration, X-rays are focused through an aperture onto our sample. We have modified the aperture to also host a jet of nitrogen and control its temperature down to 170 K. The cold jet is directed to the back of an environmental cell mounted on a circuit board. Inside the cell with front and back windows (~100 nm thin), our sample is exposed to water vapour. The temperature of the sample is measured using a custom microfabricated sensor patterned using electron-beam lithography. Finally, the cold jet cools the sample only on the window exactly where we want ice nucleation to occur. An example of ice nucleation on iron containing nano-particles, ferrihydrite, in shown in the figure. This iron mineral is contained in mineral dust, which itself is assumed to be ice active. We can distinguish first, when water drops appear (blurred area), when ice first appears and grows, and measure the corresponding temperature and humidity.
This spot-cooling technique can be used for a wide range of applications and include, in part, determining ice nucleating properties of natural aerosol samples or observing chemical changes due to reaction in aerosol particles at low temperature. As a user instrument at the PolLux endstation of the SLS, this will be available to a variety of scientists in many disciplines.
This is what makes synthetic fuel go bad.
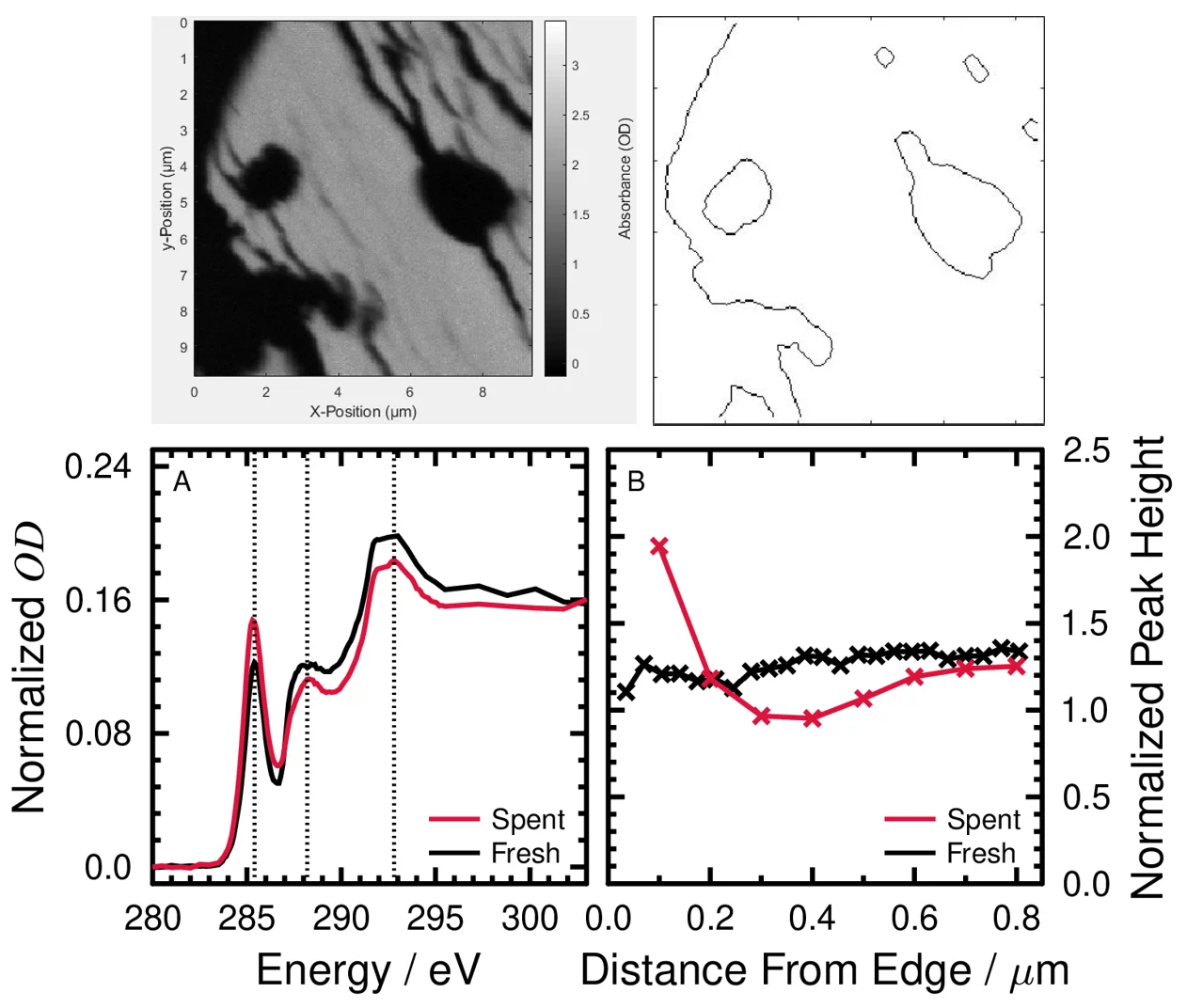
Reducing the amount of undesired products during synthetic fuel production is very important. To that end, I characterized the composition of particulate and soluble byproducts after the synthetic production of biocrude from hydrothermal processing. Hydrothermal gasification (HTG) and liquefaction (HTL) are technologies used to convert various biogenic feedstocks, such as microbial cultures, sewer sludge, black liquor and waste wood sawdust into sustainable gaseous and liquid fuels, respectively. These processes, which operate in or near supercritical water, rely on catalysts for selective fuel or gas production and heavily rely on the selective removal of unwanted carbonaceous particles or non-carbonaceous salts and compounds. The efficient recovery of brine (water and salts) is very important to protect any scavenger or catalyst that is used downstream, and relevant for recuperating costs by producing fertilizer. On the other end, understanding the formation and the fate of carbonaceous particles during such processes is of great importance to understand the impact of conditions, e.g. temperature, pressure, and time on the biocrude or the biogas production yield and composition. Indeed, the formation of carbonaceous deposits on the catalyst grains (macroscopic scale) and active sites (nanoscopic scale) is an important cause of deactivation, preventing long term operation and reducing biofuels/biogas yields and selectivity. I investigate particles and soluble compounds generated from HTL of spruce wood using scanning transmission X-ray microscopy coupled to near-edge X-ray absorption fine structure spectroscopy (STXM/NEXAFS). The desired product of HTL was the biocrude, which has great potential to be refined into liquid fuel and especially important for the aviation industry. The undesired solid particles and aqueous phase compounds contain organic carboxylic acids and unsaturated phenolic compounds. Catalysts are required for faster reactions forming targeted compounds in biocrude. I have investigated 2 mm grains that support nanometer size catalyst particles. There are carbonaceous deposits on the grain pores that can deactivate the catalysts over time, which can be quantified and avoided for better HTL performance. This would also benefit the production of aircraft fuels in next steps of fuel production since this fuel type requires nearly zero oxygen content.
Soot and oil nanoparticle emissions from aircraft engines
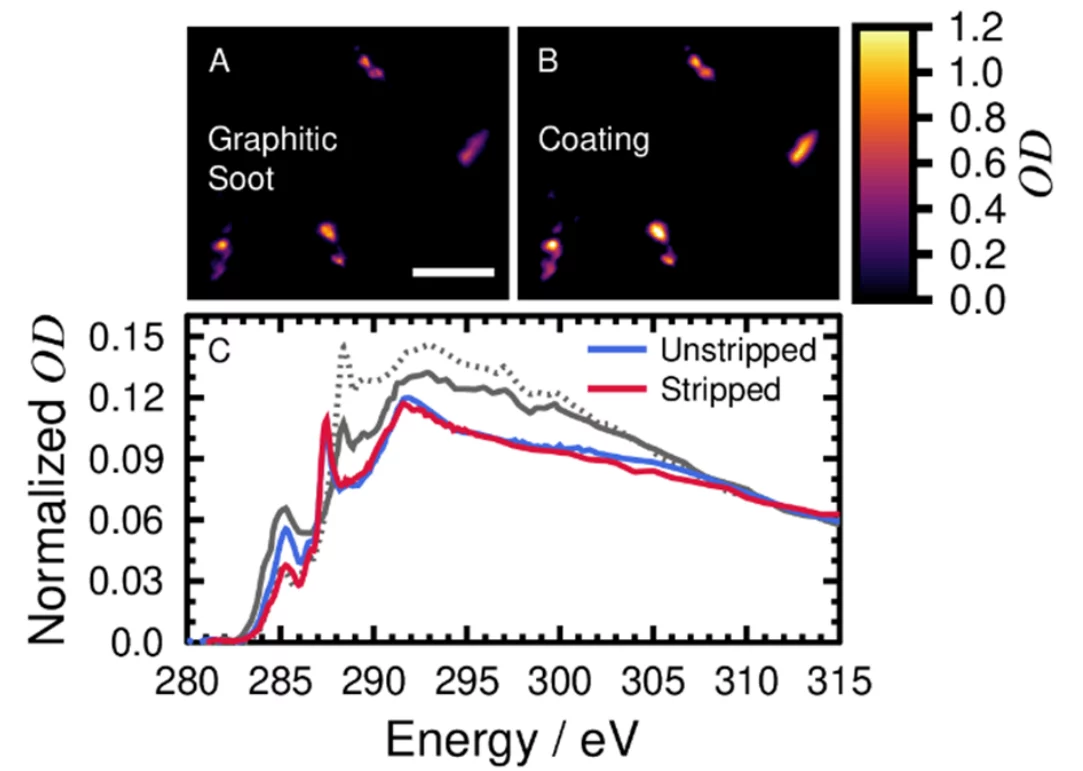
Harmful gasses and aerosol particles are a health risk when inhaled. I quantify the concentration and composition of commercial aircraft engine emissions and identify their contribution to ambient air nearby Zurich airport and to ice cloud formation. Combustion in aircraft engines generate particulate matter (PM), which can induce oxidative stress in bronchial cells, as well as pulmonary and respiratory inflammation. This is especially the case for emission during idling and taxiing engine operation. At the engine exit plane, soot is the main particulate pollutant, like at any other combustion engine. However, lubrication oil is hypothesized here to be a significant fraction of emissions, and contains additives, such as tricresyl phosphates, and metals linked to neurotoxicity. My aim is to characterize particulate emissions directly from aircraft engines by chemically distinguishing between single or mixed soot and oil particles. Single particle analysis was performed using scanning transmission X-ray microscopy coupled with near-edge X-ray absorption fine structure spectroscopy (STXM/NEXAFS). Here, X-rays were used to resolve chemical detail on a scale of tens of nanometers. In collaboration with the Zurich University of Applied Sciences (ZHAW) Centre for Aviation, I sampled particles from in-service turbofans in the silencer of the engine Test Cell at SR Technics. Engines after previous repair and overhaul are tested for their performance in the Test Cell. The silencer part of the Test Cell is a large building designed to muffle sound and safely direct emissions away from the staff and surrounding work area. Particles sampled from the silencer were directed to either a multi-orifice uniform deposition impactor (MOUDI) or an electrostatic precipitator onto suitable substrates for further analysis. X-ray optical density, OD, images of particles emitted from an aircraft engine and their characteristic NEXAFS. Soot and oxygenated carbon functions that are more organic-like are present, highly characteristic of aircraft soot. This composition indicates quinone functionalities and non-soot like aliphatic carbon. Preliminary atomic resolution transmission electron microscopy results yield an amorphous coating on the soot similar to that found in our previous research. However, this composition is not typical of soot from other combustion and urban sources that typically have a spectral signature of carboxyl functionalities (COOH). I hypothesize that atmospheric processing and oxidation will generate carboxyl functions, and that fresh aircraft soot will be identified in the vicinity of the airport using this unique chemical marker at 287.5 eV during our upcoming field campaign.
Recent Publications
-
Garner NM, Mahrt F, Top J, Tadei V, Kilchhofer K, Takahama S, et al.
Photochemistry of iron-containing secondary organic aerosol is impacted by relative humidity during formation
npj Climate and Atmospheric Science. 2025; 8(1): 246 (9 pp.). https://doi.org/10.1038/s41612-025-01109-6
DORA PSI -
Kilchhofer K, Ammann M, Torrent L, Cheung RKY, Alpert PA
Copper accelerates photochemically induced radical chemistry of iron-containing secondary organic aerosol (SOA)
Atmospheric Chemistry and Physics. 2025; 25(14): 8061-8086. https://doi.org/10.5194/acp-25-8061-2025
DORA PSI -
Mishra A, Kilchhofer K, Iezzi L, Pöschl U, Alpert PA, Ammann M, et al.
Photochemical degradation of iron citrate in anoxic viscous films enhanced by redox cascades
ACS Earth and Space Chemistry. 2025; 9(3): 689-698. https://doi.org/10.1021/acsearthspacechem.4c00364
DORA PSI -
Alpert PA, Corral Arroyo P, Ammann M
Microanalytical studies of multiphase reactions and particle aging
In: Conny JM, Buseck PR, eds. Microanalysis of Atmospheric Particles Microanalysis of atmospheric particles: techniques and applications. Geophysical monograph series. Hoboken: Wiley; 2024:201-222. https://doi.org/10.1002/9781119554318.ch10
DORA PSI -
Ammann M, Alpert PA, Artiglia L, Bao F, Bartels-Rausch T, Ospina JFF, et al.
Multiphase chemistry in the atmosphere
Chimia. 2024; 78(11): 754-761. https://doi.org/10.2533/chimia.2024.754
DORA PSI -
Decker ZCJ, Alpert PA, Ammann M, Anet JG, Bauer M, Cui T, et al.
Emission and formation of aircraft engine oil ultrafine particles
ACS ES&T Air. 2024; 1(12): 1662-1672. https://doi.org/10.1021/acsestair.4c00184
DORA PSI -
Sellegri K, Simó R, Wang B, Alpert PA, Altieri K, Burrows S, et al.
Influence of open ocean biogeochemistry on aerosol and clouds: recent findings and perspectives
Elementa: Science of the Anthropocene. 2024; 12(1): 00058 (35 pp.). https://doi.org/10.1525/elementa.2023.00058
DORA PSI -
Testa B, Durdina L, Alpert PA, Mahrt F, Dreimol CH, Edebeli J, et al.
Soot aerosols from commercial aviation engines are poor ice-nucleating particles at cirrus cloud temperatures
Atmospheric Chemistry and Physics. 2024; 24(7): 4537-4567. https://doi.org/10.5194/acp-24-4537-2024
DORA PSI -
Alpert PA, Bernard F, Connolly P, Crabeck O, George C, Kaiser J, et al.
Application of simulation chambers to investigate interfacial processes
In: Doussin J-F, Fuchs H, Kiendler-Scharr A, Seakins P, Wenger J, eds. A practical guide to atmospheric simulation chambers. Cham: Springer Nature; 2023:293-330. https://doi.org/10.1007/978-3-031-22277-1_8
DORA PSI -
Armanious A, Wang H, Alpert PA, Medaglia C, Peydayesh M, Zwygart AC-A, et al.
Trapping virus-loaded aerosols using granular material composed of protein nanofibrils and iron oxyhydroxides nanoparticles
Frontiers in Soft Matter. 2023; 3: 1143958 (10 pp.). https://doi.org/10.3389/frsfm.2023.1143958
DORA PSI -
Knopf DA, Alpert PA
Atmospheric ice nucleation
Nature Reviews Physics. 2023; 5: 203-217. https://doi.org/10.1038/s42254-023-00570-7
DORA PSI -
Yao Y, Alpert PA, Zuend A, Wang B
Does liquid-liquid phase separation impact ice nucleation in mixed polyethylene glycol and ammonium sulfate droplets?
Physical Chemistry Chemical Physics. 2023; 25(1): 80-95. https://doi.org/10.1039/d2cp04407b
DORA PSI -
Alpert PA, Boucly A, Yang S, Yang H, Kilchhofer K, Luo Z, et al.
Ice nucleation imaged with X-ray spectro-microscopy
Environmental Science: Atmospheres. 2022; 2(3): 335-351. https://doi.org/10.1039/D1EA00077B
DORA PSI -
Alpert PA, Kilthau WP, O'Brien RE, Moffet RC, Gilles MK, Wang B, et al.
Ice-nucleating agents in sea spray aerosol identified and quantified with a holistic multimodal freezing model
Science Advances. 2022; 8(44): eabq6842 (11 pp.). https://doi.org/10.1126/sciadv.abq6842
DORA PSI -
Corral Arroyo P, David G, Alpert PA, Parmentier EA, Ammann M, Signorell R
Amplification of light within aerosol particles accelerates in-particle photochemistry
Science. 2022; 376(6590): 293-296. https://doi.org/10.1126/science.abm7915
DORA PSI -
Alpert PA, Dou J, Corral Arroyo P, Schneider F, Xto J, Luo B, et al.
Photolytic radical persistence due to anoxia in viscous aerosol particles
Nature Communications. 2021; 12(1): 1769 (8 pp.). https://doi.org/10.1038/s41467-021-21913-x
DORA PSI -
Dou J, Alpert PA, Corral Arroyo P, Luo B, Schneider F, Xto J, et al.
Photochemical degradation of iron(III) citrate/citric acid aerosol quantified with the combination of three complementary experimental techniques and a kinetic process model
Atmospheric Chemistry and Physics. 2021; 21(1): 315-338. https://doi.org/10.5194/acp-21-315-2021
DORA PSI -
Knopf DA, Barry KR, Brubaker TA, Jahl LG, Jankowski KA, Li J, et al.
Aerosol–ice formation closure: a Southern Great Plains field campaign
Bulletin of the American Meteorological Society. 2021; 102(10): E1952-E1971. https://doi.org/10.1175/BAMS-D-20-0151.1
DORA PSI -
Forster J-D, Gurk C, Lamneck M, Tong H, Ditas F, Steimer SS, et al.
MIMiX: a multipurpose in situ microreactor system for X-ray microspectroscopy to mimic atmospheric aerosol processing
Atmospheric Measurement Techniques. 2020; 13(7): 3717-3729. https://doi.org/10.5194/amt-13-3717-2020
DORA PSI -
Knopf DA, Alpert PA, Zipori A, Reicher N, Rudich Y
Stochastic nucleation processes and substrate abundance explain time-dependent freezing in supercooled droplets
npj Climate and Atmospheric Science. 2020; 3: 2 (9 pp.). https://doi.org/10.1038/s41612-020-0106-4
DORA PSI -
Mahrt F, Alpert PA, Dou J, Grönquist P, Arroyo PC, Ammann M, et al.
Aging induced changes in ice nucleation activity of combustion aerosol as determined by near edge X-ray absorption fine structure (NEXAFS) spectroscopy
Environmental Science: Processes and Impacts. 2020; 22(4): 895-907. https://doi.org/10.1039/C9EM00525K
DORA PSI -
Alpert PA, Corral Arroyo P, Dou J, Krieger UK, Steimer SS, Förster J-D, et al.
Visualizing reaction and diffusion in xanthan gum aerosol particles exposed to ozone
Physical Chemistry Chemical Physics. 2019; 21(37): 20613-20627. https://doi.org/10.1039/C9CP03731D
DORA PSI -
Corral Arroyo P, Aellig R, Alpert PA, Volkamer R, Ammann M
Halogen activation and radical cycling initiated by imidazole-2-carboxaldehyde photochemistry
Atmospheric Chemistry and Physics. 2019; 19(16): 10817-10828. https://doi.org/10.5194/acp-19-10817-2019
DORA PSI -
Dou J, Luo B, Peter T, Alpert PA, Corral Arroyo P, Ammann M, et al.
Carbon dioxide diffusivity in single, levitated organic aerosol particles
Journal of Physical Chemistry Letters. 2019; 10(15): 4484-4489. https://doi.org/10.1021/acs.jpclett.9b01389
DORA PSI -
Liati A, Schreiber D, Alpert PA, Liao Y, Brem BT, Corral Arroyo P, et al.
Aircraft soot from conventional fuels and biofuels during ground idle and climb-out conditions: electron microscopy and X-ray micro-spectroscopy
Environmental Pollution. 2019; 247: 658-667. https://doi.org/10.1016/j.envpol.2019.01.078
DORA PSI -
Alpert PA, Corral Arroyo P, Dou J, Krieger UK, Steimer SS, Förster J-D, et al.
Imaging molecular reaction and diffusion in organic aerosol particles
Microscopy and Microanalysis. 2018; 24(Suppl. 2): 496-497. https://doi.org/10.1017/S143192761801471X
DORA PSI -
Corral Arroyo P, Bartels-Rausch T, Alpert PA, Dumas S, Perrier S, George C, et al.
Particle-phase photosensitized radical production and aerosol aging
Environmental Science and Technology. 2018; 52(14): 7680-7688. https://doi.org/10.1021/acs.est.8b00329
DORA PSI -
Knopf DA, Alpert PA, Wang B
The role of organic aerosol in atmospheric ice nucleation: a review
ACS Earth and Space Chemistry. 2018; 2(3): 168-202. https://doi.org/10.1021/acsearthspacechem.7b00120
DORA PSI -
Kong X, Wolf MJ, Roesch M, Thomson ES, Bartels-Rausch T, Alpert PA, et al.
A continuous flow diffusion chamber study of sea salt particles acting as cloud nuclei: deliquescence and ice nucleation
Tellus, Series B: Chemical and Physical Meteorology. 2018; 70(1): 1463806 (11 pp.). https://doi.org/10.1080/16000889.2018.1463806
DORA PSI -
Alpert PA, Ciuraru R, Rossignol S, Passananti M, Tinel L, Perrier S, et al.
Fatty acid surfactant photochemistry results in new particle formation
Scientific Reports. 2017; 7(1): 12693 (11 pp.). https://doi.org/10.1038/s41598-017-12601-2
DORA PSI -
Alpert P, Archibald A, Arnold S, Ashworth K, Brown S, Campbell S, et al.
New tools for atmospheric chemistry: General discussion
Faraday Discussions. 2017; 200: 663-691. https://doi.org/10.1039/c7fd90041d
DORA PSI -
Brüggemann M, Hayeck N, Bonnineau C, Pesce S, Alpert PA, Perrier S, et al.
Interfacial photochemistry of biogenic surfactants: a major source of abiotic volatile organic compounds
Faraday Discussions. 2017; 200: 59-74. https://doi.org/10.1039/c7fd00022g
DORA PSI -
China S, Alpert PA, Zhang B, Schum S, Dzepina K, Wright K, et al.
Ice cloud formation potential by free tropospheric particles from long-range transport over the Northern Atlantic Ocean
Journal of Geophysical Research: Atmospheres. 2017; 122(5): 3065-3079. https://doi.org/10.1002/2016JD025817
DORA PSI -
Coluzza I, Creamean J, Rossi MJ, Wex H, Alpert PA, Bianco V, et al.
Perspectives on the future of ice nucleation research: research needs and unanswered questions identified from two international workshops
Atmosphere. 2017; 8(8): 138 (28 pp.). https://doi.org/10.3390/atmos8080138
DORA PSI