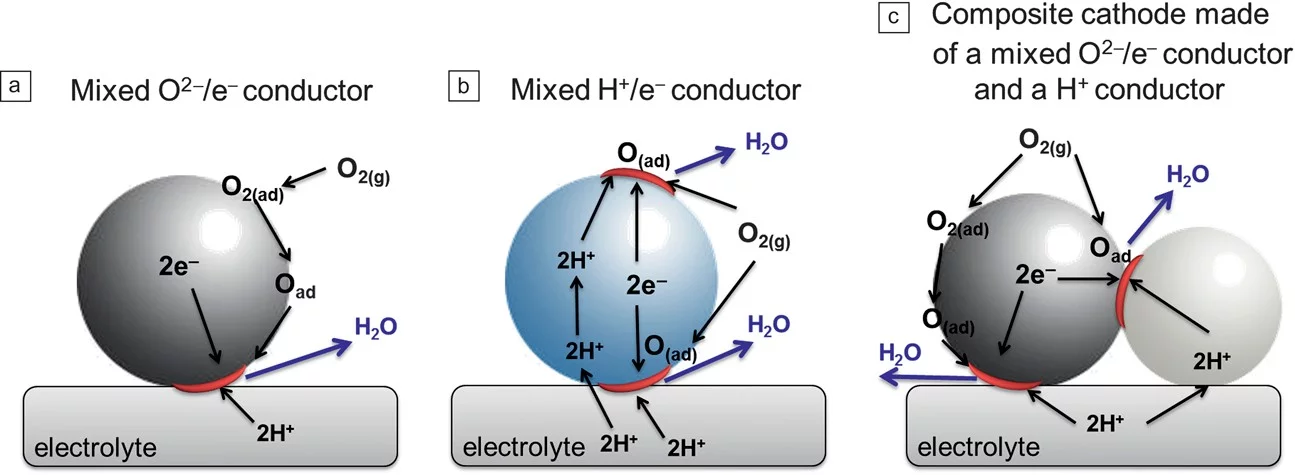
The need for reducing the operating temperature of solid-oxide fuel cells (SOFCs) imposed by cost reduction has pushed significant progress in fundamental understanding of the individual components, as well as materials innovation and device engineering. Proton-conducting oxides have emerged as potential alternative electrolyte materials to oxygen-ion conducting oxides for operation at low and intermediate temperatures. This article describes major recent developments in electrolytes, electrodes, and complete fuel cell performance for SOFCs based on proton-conducting electrolytes. Although the performance of such fuel cells is still relatively modest, significant improvements in the power density output have been made during the last couple of years, and this trend is expected to continue.
Keywords: Solid oxide full cells; Proton conductors; Ion conductors;
Facility: ENE, Electrochemistry, LMX, Thin Films and Interfaces
Reference:*E. Fabbri, A. Magrasó, and D. Pergolesi, MRS Bulletin, *39, 792-797 (2014)
Read full article: here
Facility: ENE, Electrochemistry, LMX, Thin Films and Interfaces
Reference:*E. Fabbri, A. Magrasó, and D. Pergolesi, MRS Bulletin, *39, 792-797 (2014)
Read full article: here