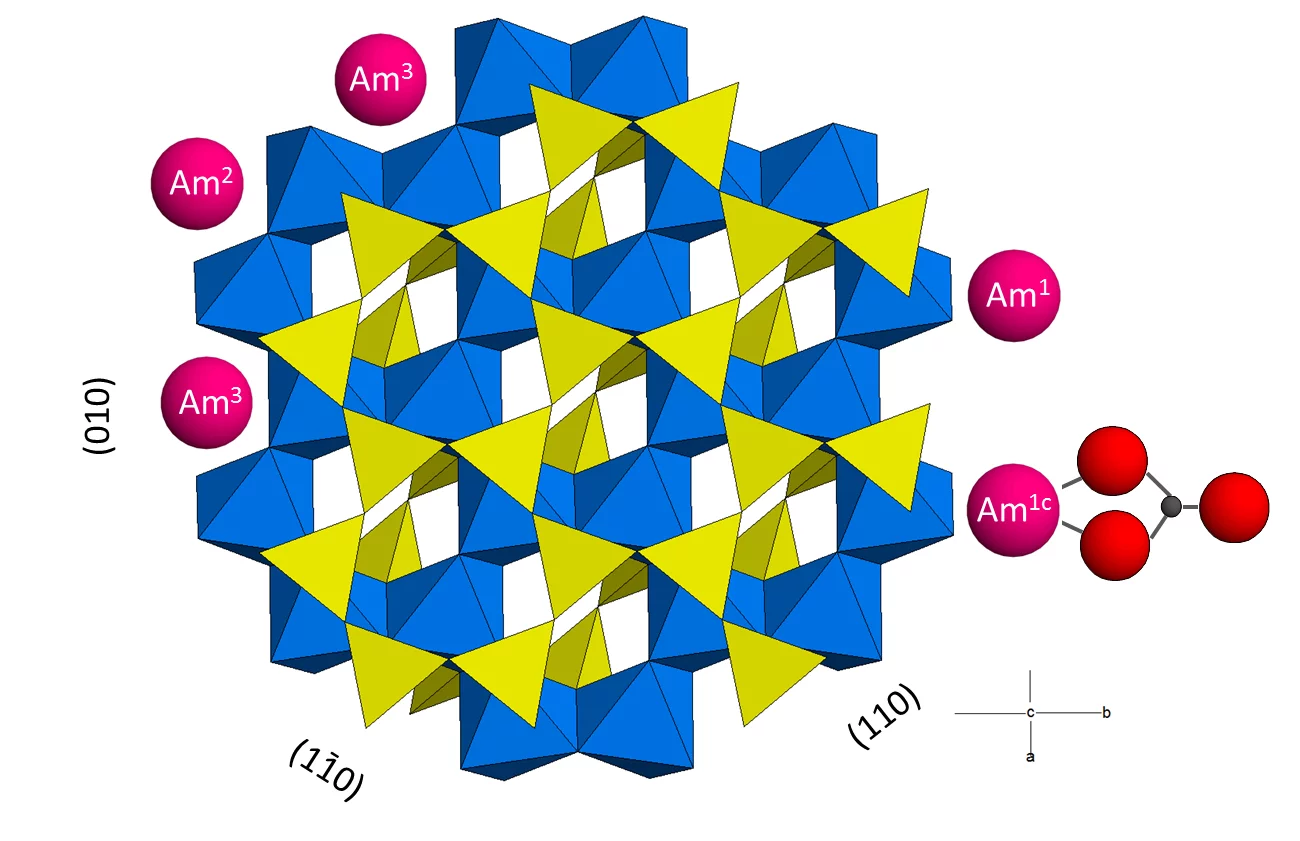
The credibility of long-term safety assessments of radioactive waste repositories may be greatly enhanced by a molecular level understanding of the sorption processes onto individual minerals present in the near- and far-fields. In this study we couple macroscopic sorption experiments to surface complexation modelling and spectroscopic investigations, including extended X-ray absorption fine structure (EXAFS) and time-resolved laser fluorescence spectroscopies (TRLFS), to elucidate the uptake mechanism of trivalent lanthanides and actinides (Ln/AnIII) by montmorillonite in the absence and presence of dissolved carbonate. Based on the experimental sorption isotherms for the carbonate-free system, the previously developed sorption model needed to be complemented with an additional surface complexation reaction onto a weak site. The sorption isotherms in the presence of carbonate required refinement of the previously published model by reducing the strong-site capacity and by adding the formation of Ln/AnIII-carbonato complexes both on strong and weak sites. EXAFS spectra of selected Am samples and TRLFS spectra of selected Cm samples corroborate the model assumptions by showing the existence of different surface complexation sites and evidencing the formation of Ln/AnIII carbonate surface complexes (Figure 1). In the absence of carbonate and at low loadings, Ln/AnIII form strong inner-sphere complexes through binding to three Al(O,OH)6 octahedra, most likely by occupying vacant sites in the octahedral layers of montmorillonite, which are exposed on {010} and {110} edge faces. At higher loadings, Ln/AnIII binds to only one Al octahedron, forming a weaker, edge-sharing surface complex. In the presence of carbonate, we identified a ternary mono- or dicarbonato Ln/AnIII complex binding directly to one Al(O,OH)6 octahedron, revealing that type-A ternary complexes form with the one or two carbonate groups pointing away from the surface into the solution phase. Within the spectroscopically observable concentration range these complexes could only be identified on the weak sites, in line with the small strong site capacity suggested by the sorption model. When the solubility of carbonates was exceeded, formation of an Am carbonate hydroxide could be identified. The excellent agreement between the thermodynamic model parameters developed by fitting the macroscopic data, and the spectroscopically identified mechanisms, demonstrates the mature state of the 2SPNE SC/CE model for predicting and quantifying the retention of Ln/AnIII elements by montmorillonite-rich clay rocks.
Contact
Dr. Maria Marques
Clay Sorption Mechanisms Group
Laboratory for Waste Management (LES)
Paul Scherrer Institut
Telephone: +41 56 310 2310
E-mail: maria.marques@psi.ch
Original Publication
Sorption of trivalent lanthanides and actinides onto montmorillonite: Macroscopic, thermodynamic and structural evidence for ternary hydroxo and carbonato surface complexes on multiple sorption sites
M. Marques Fernandes, A.C. Scheinost and B. Baeyens (2016)
Water Research Vol. 99, pp. 74–82
DOI: http://dx.doi.org/10.1016/j.watres.2016.04.046