Electrolyzers show great promise for compensating the variable energy production from renewable resources by producing the versatile energy vector hydrogen; however, the development of efficient, robust and economically viable electrolyzers is still facing important scientific and technological challenges. Oxygen electrocatalysis plays a pivotal role in the development of electrolyzers since the anodic reaction, the oxygen evolution reaction (OER), represents one of the main sources of overpotential. Perovskite oxides have emerged as promising anodic electrodes for alkaline water electrolyzers, and an increasing amount of studies have been recently published about perovskite OER catalysts. Particularly, many studies have dedicated in finding “activity descriptors” which means the key perovskite properties governing the OER activity. While, the first studies on perovskite catalysts have been mostly focused on the correlation of bulk perovskite properties with the OER activity, recent research efforts have been more and more directed towards the understanding of the electrode/electrolyte interface. The growing attention towards interface properties has fostered the development of surface sensitive techniques, particularly in situ and under operando conditions, such as near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS).
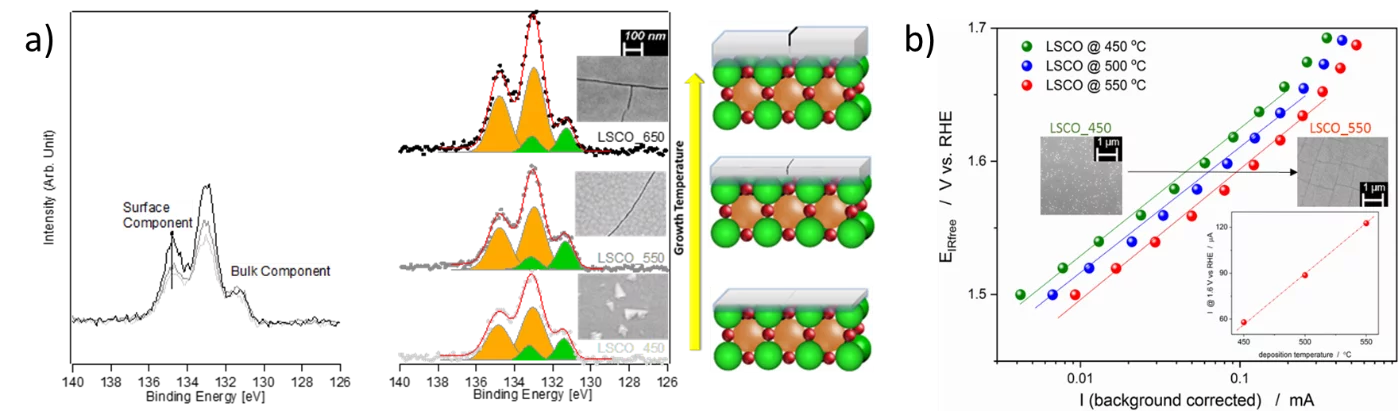
PSI researchers have studied the influence of surface segregation on the OER activity for the, La0.2Sr0.8CoO3-d (LSCO) perovskite, one of the most active perovskite towards the OER in alkaline electrolyte. LSCO has been produced in the form of thin films by means of pulsed laser deposition (PLD) using different deposition temperatures (between 350 and 650 °C). The morphology of the films has been characterized by means of SEM and atomic force microscopy (AFM) while the crystalline structures have been checked with X-ray diffraction (XRD). NAP-XPS has been used to check the surface composition of perovskite films synthesized at different temperatures, showing that high temperature favors strontium segregation in the topmost layers. However, strontium forms surface compounds that are efficiently removed when the catalyst surface is in contact with water. Indeed, NAP-XPS measurements revealed that after immersion in water, LSCO shows a cobalt rich surface, which is very active for the OER. More generally, higher deposition temperature in the 450-550°C range, translates into higher OER activity for the LSCO catalysts. Indeed, higher the perovskite synthesis temperature the more strontium segregation occurs on the surface. However, being the segregated strontium compounds soluble in water, they are easily removed when the surface of the electrode is in contact with the electrolyte, leading to the exposure of cobalt enriched layers very active for the OER.
Contact
Dr. Emiliana Fabbri, Scientist
Dr. Luca Artiglia, Scientist
Prof. Dr. Thomas J. Schmidt, Group Leader and Department Head
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 27 95
E-mail: emiliana.fabbri@psi.ch; luca.artiglia@psi.ch; thomasjustus.schmidt@psi.ch
Original Publication
Surface segregation acts as surface engineering for the oxygen evolution reaction on perovskite oxides in alkaline media
Anthony Boucly, Emiliana Fabbri, Luca Artiglia, Xi Cheng, Daniele Pergolesi, Markus Ammann, Thomas J. Schmidt
Chemistry of Materials
DOI: 10.1021/acs.chemmater.0c01396
Acknowledgement
The authors would like to thank Innosuisse and the Swiss Competence Center for Energy Research (SCCER) Heat & Electricity Storage.