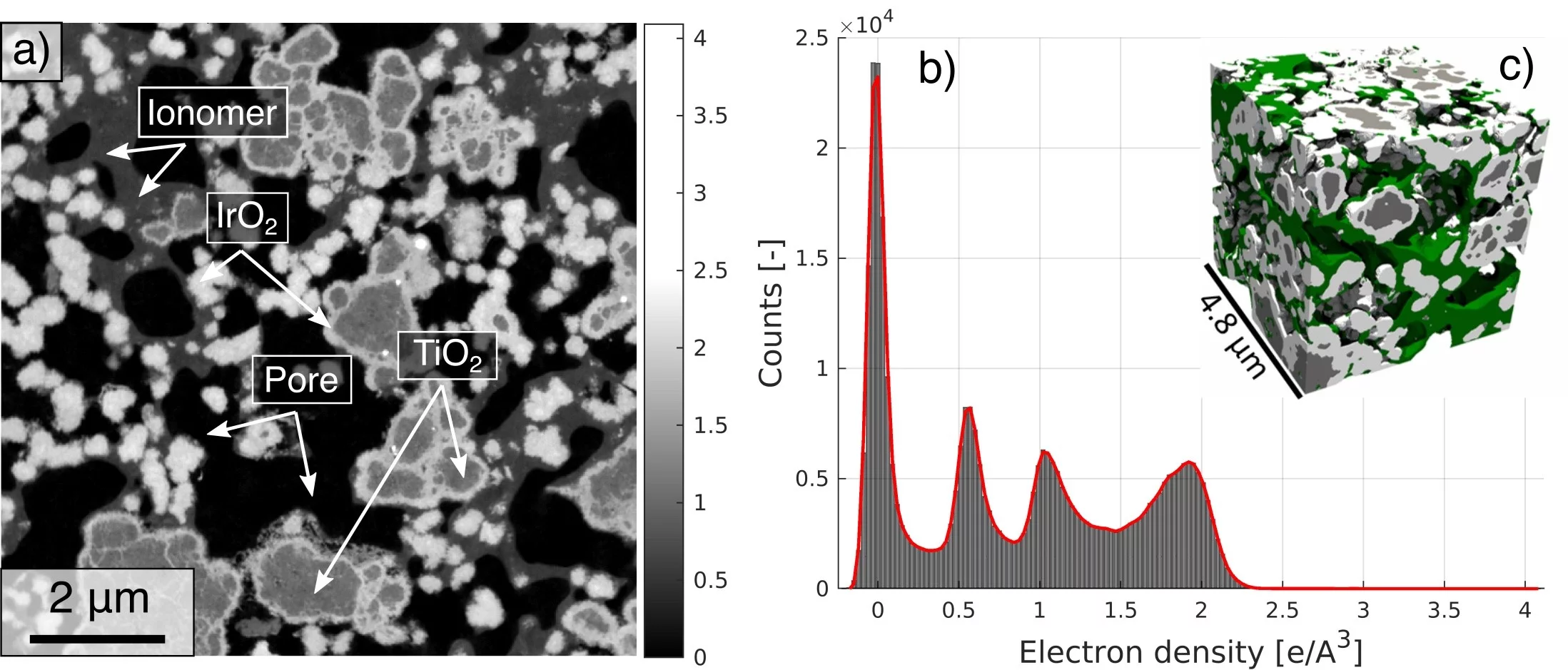
Reducing precious metal loading in the anodic catalyst layer (CL) is indispensable for lowering capital costs and enabling the widespread adoption of polymer electrolyte water electrolysis (PEWE). This work presents the first three-dimensional reconstruction of a TiO2-supported IrO2 based core shell catalyst layer, using high-resolution X-ray ptychographic tomography at cryogenic temperature of 90 K. The high data quality and phase sensitivity of the technique have allowed the reconstruction of all four phases namely pore space, IrO2, TiO2 support matrix and the ionomer network, the latter of which has proven to be a challenge in the past.
The analyzed volume of 9x9x7 µm3 is proved to be representative for all phases. The phase fraction calculations present a discrepancy to the expected values derived from the catalyst layer ink composition. The overestimation of segmented IrO2 volume fraction is caused by the presence of nanoporous IrO2 as confirmed by SEM images. The analysis of the continuous pore and particle size distribution reveals that the void phase presents a broader size distribution compared to the other phases.
Results show that the pore phase has the least tortuosity while the ionomer with 7.1 presents the highest values. The effective conductivities computed from the tomographic data are comparable with previous experimentally reported values and indicate that, in the structure with a non-swollen ionomer, the ionic conductivity is the limiting component, even if the ionomer is in a well-conducting state.
The analysis of the CL ionic and electronic conductivity shows that for a dry CL, the ionic conductivity is orders of magnitudes lower than the electronic conductivity. Varying the electronic conductivity of the support phase by simulations, reveals that the conductivity of the support does not have a considerable impact on the overall CL electrical conductivity.
This study provided not only insights into state-of-the-art catalyst layers via synchrotron-based PXCT and guidance of rational catalyst layer design but also provides a novel tool for analysis of future generation low-loaded CL microstructures.
Contact
Dr. Felix N. Büchi
Head Fuel Cell Systems and Diagnostics Group
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 24 10
E-mail: felix.buechi@psi.ch
Original Publication
Understanding the microstructure of a core-shell based anode catalyst layer for polymer electrolyte water electrolysis
S. De Angelis, T. Schuler, M. Sabharwal, M. Holler, M. Guizar-Sicairos, E. Müller, F.N. Büchi
Sci Rep 13, 4280 (2023).
DOI: 10.1038/s41598-023-30960-x
Acknowledgement
The authors would like to thank the Swiss federal office of Energy (SFOE, grant no. SI/501331-01) for contributing funding towards this project. We express our gratitude to Umicore (Belgium) for providing catalyst material for this study. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland, for the provision of synchrotron radiation beamtime at the cSAXS beamline of the SLS. Finally, we want to thank Carl Cesar Weber for his support during the beamtime preparation.