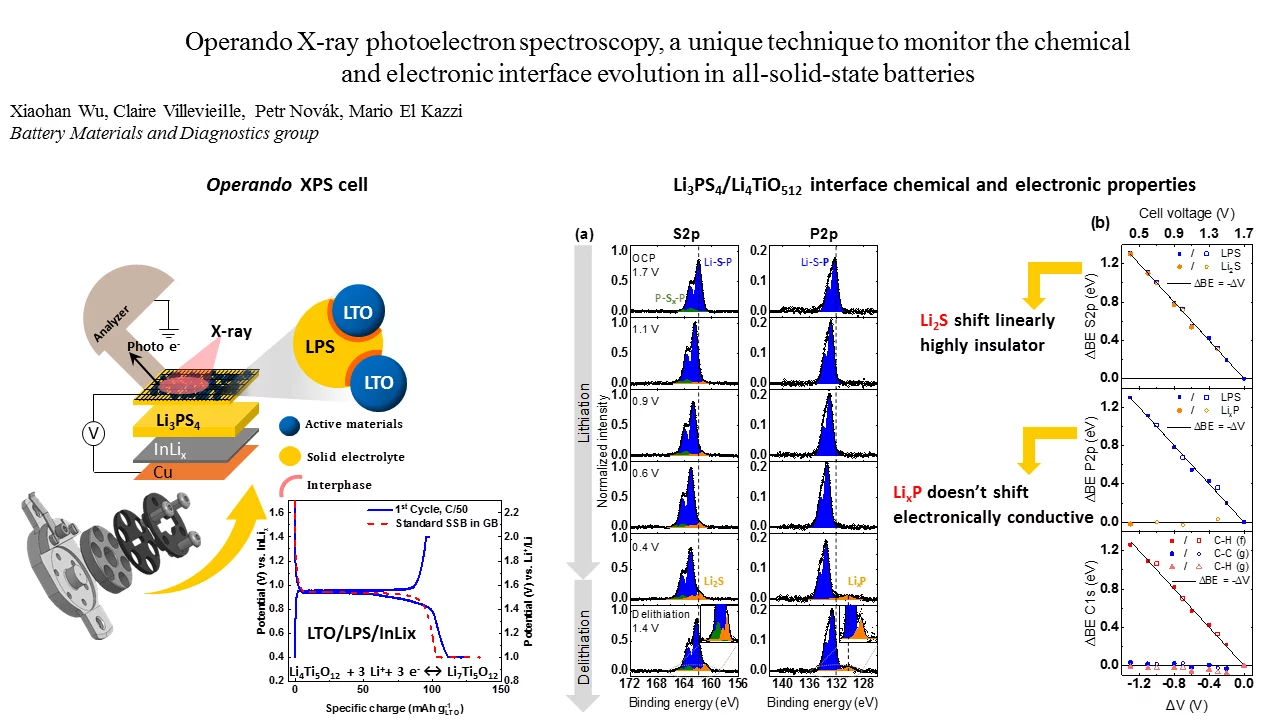
Degradation of the solid-electrolyte interface occurring during cycling is currently one of the most challenging issues for the development of all-solid-state batteries. Here we designed a unique electrochemical custom made cell for operando X-ray photoelectron spectroscopy (XPS) capable of maintaining high mechanical pressure with reliable electrochemistry and able to monitor in real-time the surface (electro-)chemical reactivity at the interfaces between the different composite.
The (electro-)chemical reactivity and electronic properties across electrified solid-solid interfaces critically determine the functionality of solid-state electrochemical devices. To date, questions still arise in the scientific community due to the limitations of surface-sensitive characterization techniques, especially in post-mortem mode. Particularly, parameters such as the electrochemical stability of the solid electrolyte (SE) as well as the ionic and electronic percolation within the composite electrode are essential in order to push all-solid-state lithium-ion batteries (SSBs) towards practical applications. In this work, we highlight operando X-ray photoelectron spectroscopy (XPS) as a straightforward method to monitor the SE reduction within a composite electrode consisting of Li4Ti5O12 (LTO) as the electrode active material, (Li2S)3-P2S5 (LPS) as the SE and vapor-grown carbon fiber as the conductive additive under real working conditions. The results clarify previous discrepancies between experimental and theoretical results regarding the anodic stability limit of LPS and also reveal the limitations of post-mortem XPS measurements that can potentially lead to misinterpretations. We identify the reduction process of LPS which starts at 1.7 V vs. Li+/Li (1.1 V vs. InLix), resolved as a two-step process accompanied by the formation of an electronically insulator Li2S and electronically conductive LixP species. Furthermore, we propose in our study a fundamental physical model to underline the effect of the external cell voltage on the core levels binding energy shifts which provides a direct contactless measurement of the local surface potentials of the different particles. Thanks to that, the operando methodology resolves the LTO redox activity and the distribution of conductive and non-conductive LTO species as a function of lithiation. This allows a deeper understanding of both the lithium-ion transport through the composite electrode and the characteristic metal-insulator transition associated with LTO. Finally, we discuss how the interfacial reactivity between LPS and LTO impacts the electrochemical performance of SSBs. Our novel operando XPS development paves the way for an accurate interpretation of the core levels peaks and to the assignment of the SE byproducts leading to a better description of the interfacial reaction mechanism in SLiB.
Contact
Dr. Mario El Kazzi, Group Leader of Battery Materials and Diagnostics
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 51 49
E-mail: mario.el-kazzi@psi.ch
Original Publications
Insights into the chemical and electronic interface evolution of Li4Ti5O12 cycled in Li2S–P2S5 enabled by operando X-ray photoelectron spectroscopy
Xiaohan Wu, Claire Villevieille, Petr Novák, Mario El Kazzi
Journal of Materials Chemistry A
DOI: 10.1039/C9TA14147B
Monitoring the chemical and electronic properties of electrolyte–electrode interfaces in all-solid-state batteries using operando X-ray photoelectron spectroscopy
Xiaohan Wu, Claire Villevieille, Petr Novák, Mario El Kazzi
Physical Chemistry Chemical Physics
DOI: 10.1039/C8CP01213J