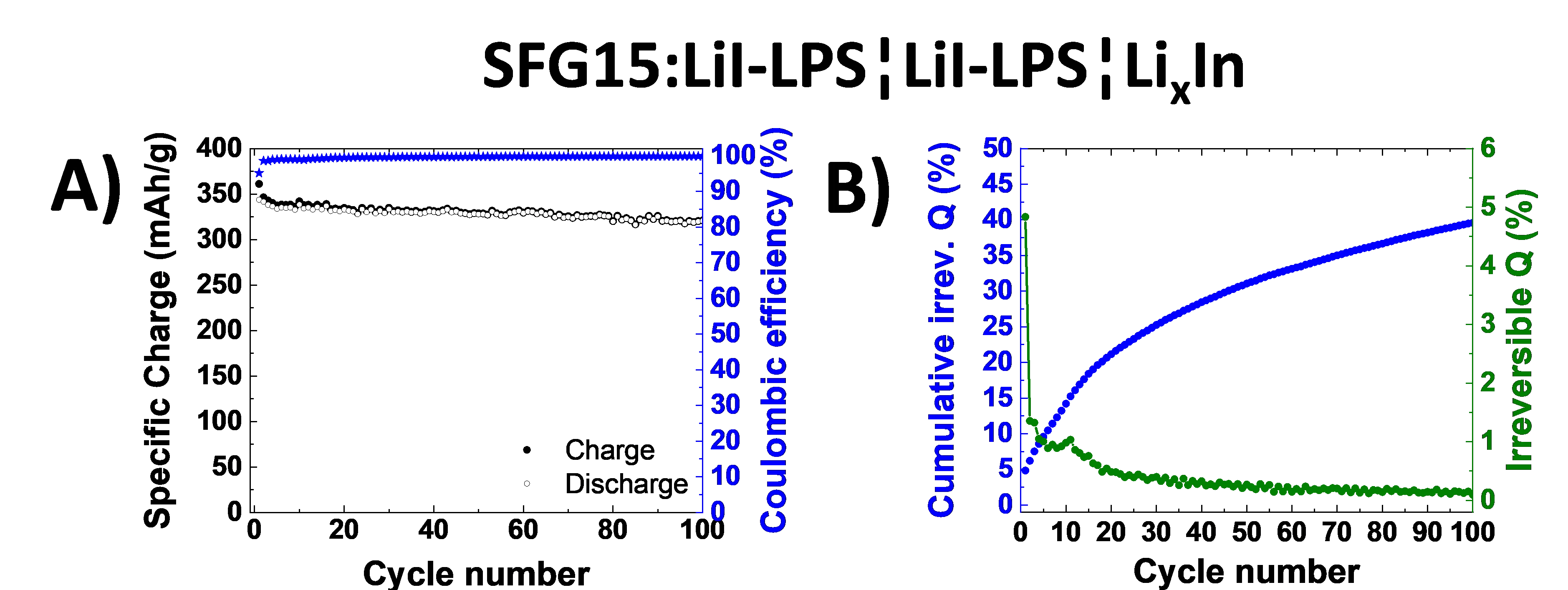
All-solid-state lithium ion batteries represent a promising battery technology for boosting the volumetric energy density and promising a superior safety. In this study excellent cycling stability of graphite anode material have been demonstrated in combination with sulfide-based solid electrolyte. Furthermore we evaluated the stability of the graphite-electrolyte interface by analyzing the normalized cumulative irreversible charge during cycling experiments.
All-solid-state lithium ion batteries could offer the great opportunity of enhanced safety, longer lifetime and higher energy density thanks to the replacement of the volatile and flammable organic liquid electrolyte by a non-flammable solid electrolyte. The sulfide based materials are among the most attractive solid electrolytes as they offer the highest ionic conductivities at room temperature and have low grain boundary resistance. Besides, the sulfide solid electrolytes can be cold pressed; thus, they can be more easily processed than their oxide or phosphate counterparts. However, their very narrow electrochemical stability window limits the ion transport across the solid electrolyte-active materials interfaces. In fact, for potentials negative to 1.7 V vs. Li+/Li, a reductive process occurs leading to their decomposition into Li2S and Li3P species. In this study, we report for the first time long-term electrochemical cycling of graphite in two different sulfide-based solid electrolytes, 0.75Li2S-0.25P2S5 and 0.3LiI-0.7(0.75Li2S-0.25P2S5) denoted (LPS) and (LiI-LPS) respectively with high confining pressure. We also evaluate the stability of the graphite-solid electrolyte interface by analyzing the normalized cumulative irreversible charge during cycling experiments. We examine how, over several months of cycling, the electrochemical performance of graphite is affected by the type of the sulfide electrolyte and the particle size, as well as the type of graphite (different size and shape). For different electrolyte-electrode combinations, we report the specific charge, the coulombic efficiency, and the capacity retention during cycling. Our results demonstrate excellent cycling stability and high utilization of graphite electrodes in cells with LPS based solid electrolytes. At a given rate, the utilization of graphite can be improved by decreasing the size of the sulfide electrolyte particles. The interface of LPS to graphite is however unstable. The layer of decomposition products at the interface increases rapidly during the first cycles and then does not stop over time, following a kind of root type function. A noticeable irreversible charge consumption exists even after several months of cycling. We show that coulombic efficiencies close to 100 % in a particular cycle are not sufficient to assume good long-term cycling behavior of a cell. To this point, we propose to compare the cumulative irreversible charge for the entire cycling to avoid misinterpretation of the electrochemical cycling data. Finally, we demonstrated that optimizing the graphite electrode morphology is important to enhance the power performance of the cells.
Contact
Dr. Mario El Kazzi, Group Leader of Battery Materials and Diagnostics
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 51 49
E-mail: mario.el-kazzi@psi.ch
Original Publication
Study of Graphite Cycling in Sulfide Solid Electrolytes
Laura Höltschi, Franziska Jud, Camelia Borca, Thomas Huthwelker, Claire Villevieille, Vincent Pelé, Christian Jordy, Mario El Kazzi, Petr Novák
J. Electrochem. Soc. 167 (11), 110558 (2020)
DOI: 10.1149/1945-7111/aba36f
Acknowledgements
The authors acknowledge the financial support from SAFT.