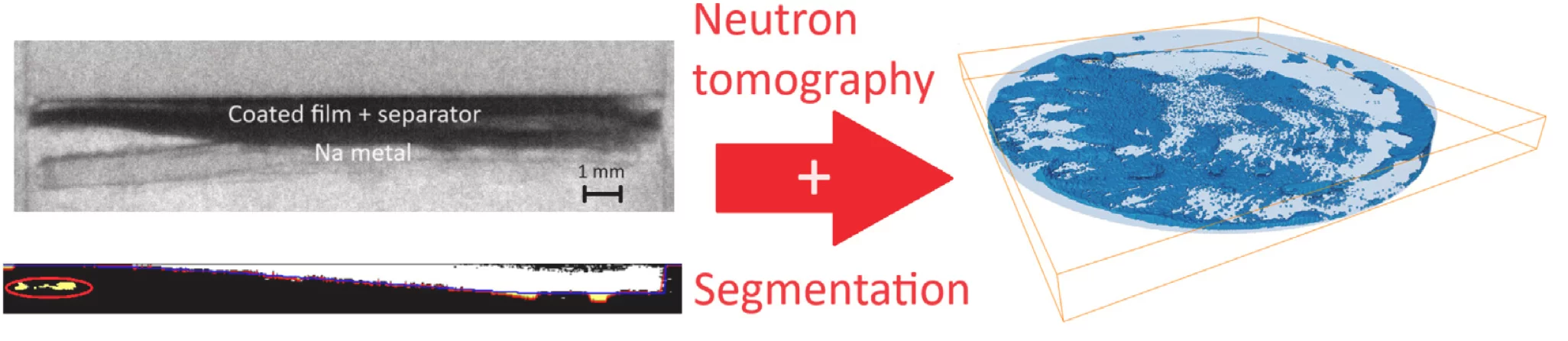
To develop durable and high-performance sodium-ion batteries, it is crucial to understand the degradation processes taking place during electrochemical cycling. This study presents the first demonstration of visualizing the effects of electrolyte degradation in sodium-ion batteries, via 2D and 3D neutron imaging thereby visualizing the degradation of the cells. The experiment was performed on a pristine sodium-based full cell, and two extensively cycled half cells differing only in the electrolyte composition. The different electrolytes, based on NaPF6 , but differing in solvent (propylene carbonate and dimethoxyethane), are chosen for their different cycling properties, allowing us to induce two different plating mechanisms. Plating phenomena highly correlate with the accumulation of so-called "dead sodium", which is detrimental to battery functioning, as it can lead to battery failure upon electrode disconnection.
We observe that the type of electrolyte is correlated to the plating mechanism, with the propylene carbonate-based cell showing bigger plating domains than the dimethoxyethane-based cell. Segmentation was performed by Python codes designed to extract features based on shape variation, as thresholding-based approaches were unsuccessful due to the relatively high incoherent scattering contribution.
The study performed on the full cell allowed us to quantify voltage-induced electrolyte degradation, while in the study on the half-cells we performed the very first demonstration of visualizing the chemical reactions occurring at the surface of the sodium metal electrode by performing 3D neutron imaging on the plating domain of the electrode.
Facility: SINQ
Reference: D. Battaglia et al, Journal of Power Sources 655, 237846 (2025)
Read full article: here