The electrochemical conversion of CO2 to ethylene offers a promising approach to expand manufacturing of commodity chemicals and fuels. Specifically, ethylene is a critical precursor for polyethylene a $240B industry. Expanding productivity at existing ethylene plants would allow for rapid growth in the sector while alleviating the time delay associated with building new refineries. Energy efficient and durable CO2 electrolysis to ethylene poses significant challenges because it requires a multistep reaction for C–C coupling, leading to multiple reaction products such as carbon monoxide, acetate, formate and ethanol.
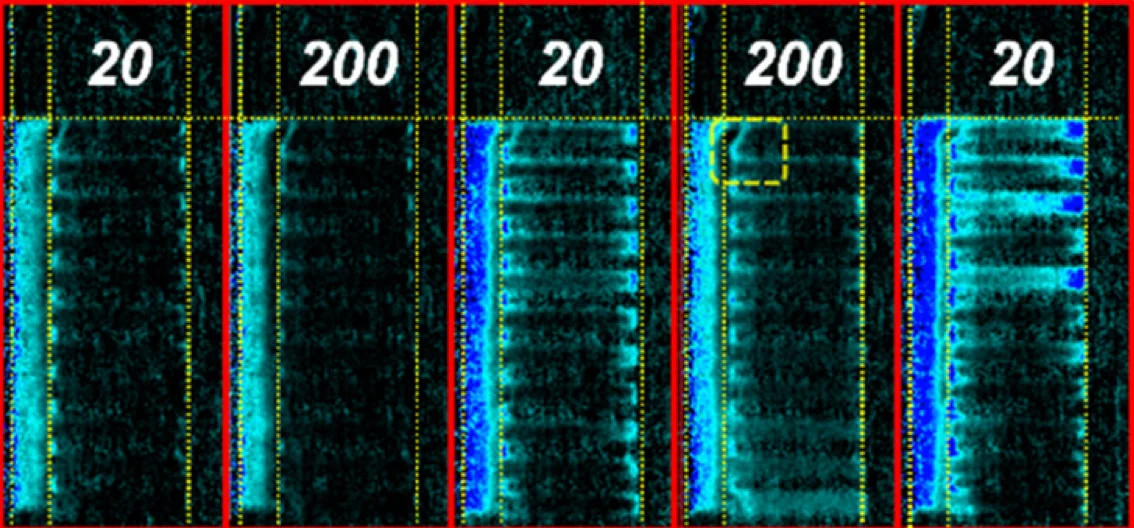
Our research addresses two short-term failure mechanisms of zero-gap CO2 electrolysis to ethylene, namely salt precipitation, and catalyst flooding, which prevent industrial adoption. We show that a low frequency current pulsing protocol can manage water movement within the electrolyzer. These relaxation pulses allow for the dissolution of precipitated salts and for liquid organic byproducts to be removed from the cathode allowing for increase durability of the electrolyzer.
Facility: SINQ
Reference: M. Marufu et al, EES Catalysis, adv. online publication (2026)
Read full article: here