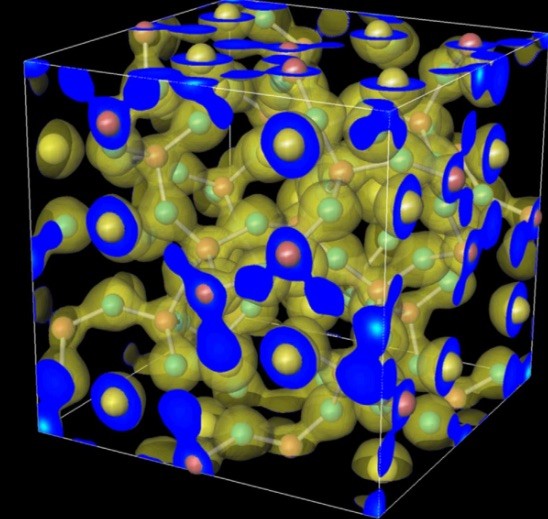
Hydride ions in oxide hosts hidden by hydroxide ions
The true oxidation state of formally ‘H?-’ ions incorporated in an oxide host is frequently discussed in connection with chemical shifts of 1H nuclear magnetic resonance spectroscopy, as they can exhibit values typically attributed to H+. Here we systematically investigate the link between geometrical structure and chemical shift of H- ?ions in an oxide host, mayenite, with a combination of experimental and ab initio approaches, in an attempt to resolve this issue. We demonstrate that the electron density near the hydrogen nucleus in an OH-? ion (formally H+ state) exceeds that in an H?- ion. This behaviour is the opposite to that expected from formal valences. We deduce a relationship between the chemical shift of H-? and the distance from the H?- ion to the coordinating electropositive cation. This relationship is pivotal for resolving H- species that are masked by various states of H+ ions.