In an underground geological repository, gas is produced by the corrosion of metallic waste or the degradation of organic waste materials. The ability of the gas to disperse in the host rock is critical to the safety of the repository. If the gas pressure exceeds the lithostatic pressure, the mechanical integrity of the host rock may be compromised. At low gas pressure, transport is controlled by gas diffusion in the saturated or partially saturated pore space. As the gas pressure increases, transport changes to a visco-capillary process. At higher pressure, dilation of the transport pathway and fracking occurs. It is essential to demonstrate that gas dissipation in the host rock exceeds the gas accumulation rate. Ideally, the gas partial pressure does not exceed diffusion-controlled conditions.
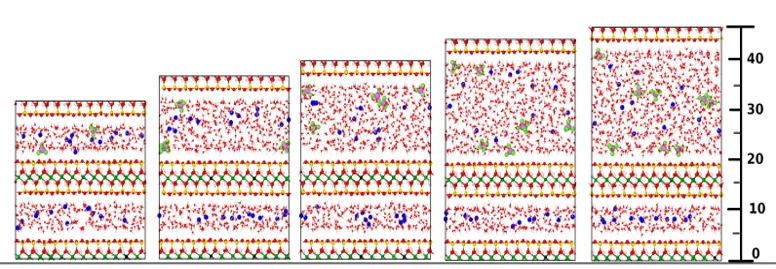
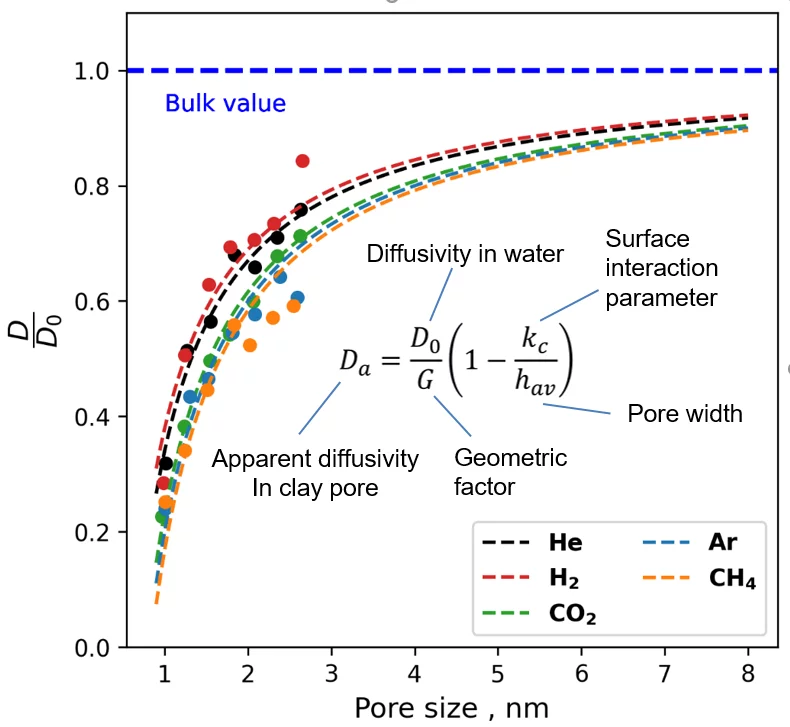
The molecular diffusion of gases at saturated and partially saturated conditions was investigated by molecular dynamics simulations as part of the EU-EURAD gas project. Figure 1 shows different systems of water-saturated smectite nanopores with dissolved gases. By analysing the molecular dynamics trajectories, the effective diffusion coefficient of gases was determined as a function of pore size (Figure 2). By combining these data with the experimentally available porosity and geometric tortuosity factor, a general relationship (equation shown in Figure 2) was derived to predict gas diffusivity based on readily available data such as gas diffusivity in bulk water (D0), average porosity (pore width) and tortuosity (geometric factor) of the host rock.
In the partially saturated state, gas migration is described by a combination of diffusive and advective transport. One of the crucial parameters controlling gas advection in nanometer-sized pores is viscosity and slip velocity. These parameters cannot be measured directly in experiments and can only be assessed indirectly by using system-specific model assumptions to interpret the experimental data.
In this study, the viscosity of gas and water films was investigated using non-equilibrium molecular dynamics.
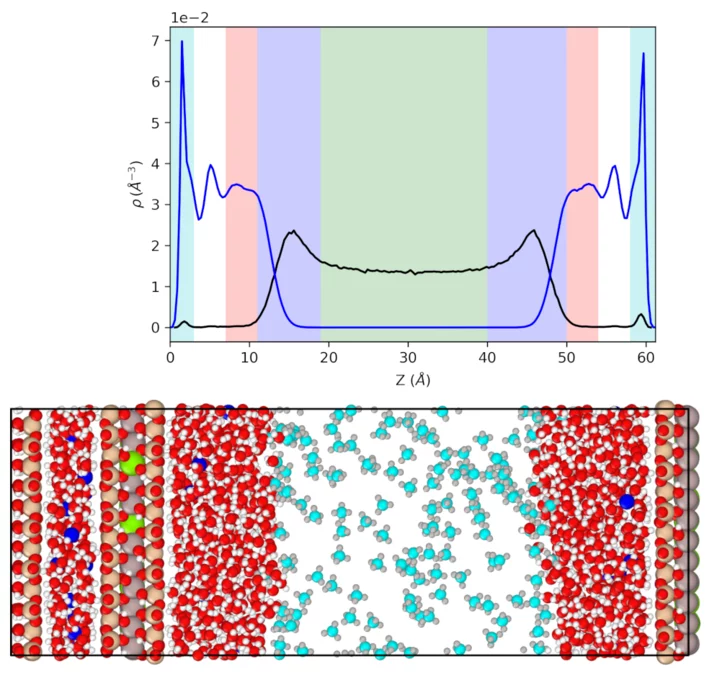
In this method, a constant force is applied to initiate Poiseuille flow. From the analysis of the velocity profiles, the gas viscosity and the slip length could be derived. For this purpose, the degree of saturation was varied in a 6 nm wide pore (Figure 3), resulting in a systematic variation of the gas-filled pore width. Based on the density profile, the bulk gas (green), the bulk liquid domain (pink) and the liquid-gas (blue) and liquid-solid (cyan) interfaces could be identified. The gas mobility and partition coefficient in each domain could be analysed. These parameters are an essential input for the reactive transport model to model the gas migration.
For the case of saturated conditions, it was shown that gas viscosity and diffusivity in nanopores follow a universal scaling relationship that can be used to predict the gas properties in confinement under arbitrary conditions.
Authors
Jerry Peprah Owusu
jerry-peprah.owusu@psi.ch
Geospheric transport [Repository safety / Nuclear Energy and Safety Division]
Sergey Churakov
sergey.churakov@psi.ch
phone: +41 56 310 4413
Head of Waste Management Laboratory [Nuclear Energy and Safety Division]
Publication List
Owusu, J. P., Karalis, K., Prasianakis, N. I., & Churakov, S. V. (2022). Mobility of dissolved gases in smectites under saturated conditions: effects of pore size, gas types, temperature, and surface interaction. Journal of Physical Chemistry C, 126(40), 17441-17455.