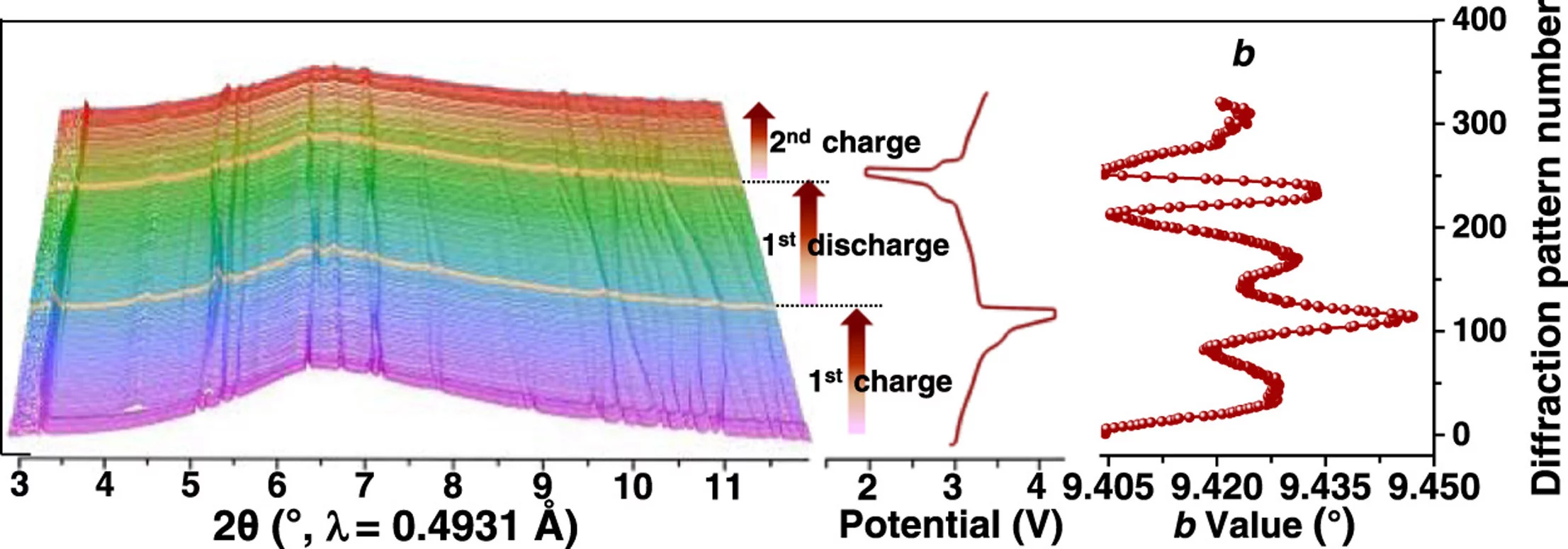
The storage of renewable energy depends largely on sustainable technologies such as sodium-ion batteries with high safety, long lifespan, low cost, and non-toxicity. Pyrophosphate Na3.32Fe2.34(P2O7)2 cathode could meet this requirement, however, its structural stability needs to be further enhanced for practical purposes. To overcome this problem, Na-deficient Na3.32Fe2.11Ca0.23(P2O7)2 with exceptional stability is prepared by Ca selective doping in this work. Operando synchrotron-based X-ray diffraction (SXRD) and in situ X-ray absorption near edge spectroscopy (XANES) results reveal that the prepared Na3.32Fe2.11Ca0.23(P2O7)2 is a single-phase solid-solution reaction with high reversibility. A strong correlation between the voltage curve and lattice parameters is deciphered for the first time. Additionally, the atomic-doping engineering strategy could significantly enhance the thermal and electrochemical stability of the electrode materials, contributing to their good structural reversibility and enhanced operational safety. Specifically, after 1000 cycles at 1 C, the Ca doped electrode achieves a high capacity retention of 81.7%, which is much better than that of the undoped electrode (15.5%).
Contact
Dr. Nicola Casati
Beamline Scientist
PSI, Laboratory for Synchrotron Radiation - Condensed Matter
Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 5346
E-mail: nicola.casati@psi.ch
Original Publication
The structural origin of enhanced stability of Na3.32Fe2.11Ca0.23(P2O7)2 cathode for Na-ion batteries
Y. Liu, Z. Wu, S. Indris, W. Hua, N.P.M. Casati, A. Tayal, M.S.D. Darma, G. Wang, Y. Liu, C. Wu, Y. Xiao, B. Zhong, X. Guo
Nano Energy 79 (2021) 105417
DOI: https://doi.org/10.1016/j.nanoen.2020.105417