Our Group
We are a highly motivated team of researchers including PhD students, postdoctoral fellows, research associates, and senior scientists dedicated to developing and improving therapeutic and diagnostic (theranostic) applications in nuclear medicine. We work interdisciplinary in the fields of chemistry, biology, pharmacy, and medicine. We welcome Master and research project students to join us and benefit from our expertise and enthusiasm for science. Our preclinical research is often conducted in close collaboration with other academic researchers, industry partners, and clinicians.
Our overarching goal is to advance targeted radionuclide therapy to address unmet biomedical needs, with a particular focus on the treatment of malignant human cancers. For more information regarding our research field, please refer to our recent book chapter providing an overview of radiopharmaceutical therapy [Grzmil et al., 2025. Radiopharmaceutical Chemistry].
Development of New Radiolabeled Drugs for Radioligand Therapy (RLT)
The broad diversity of high-affinity peptide ligands provides a valuable foundation for developing more effective and better-tolerated radiopharmaceuticals. Our research focuses on the design, optimization, and evaluation of innovative radiolabeled ligands to improve diagnostic precision and enhance the efficacy and safety of RLT. By increasing targeting specificity and reducing off-target effects, we aim to advance the theranostic use of radioligands across a range of diseases, including cancer.
Derivatized minigastrin analogues are promising drugs for targeting the cholecystokinin B receptor (CCKBR), overexpressed in various neuroendocrine tumors, including medullary thyroid cancer (MTC). Our research showed that replacing the pentaglutamic acid sequence in minigastrin with a flexible aliphatic linker enhanced internalization, tumor uptake, and blood plasma stability [Ritler et al., 2019. Bioconjug Chem]. Computational modeling revealed ionic interactions between receptor cationic residues and anionic linker residues, highlighting the role of negative charges and supporting molecular modeling for improved minigastrin analogue design. In other study, we introduced amide-to-triazole substitutions in the minigastrin analogue MG11. This “triazole scan” produced peptidomimetics with significantly improved enzymatic stability and/or CCKBR affinity [Grob et al., 2020. J Med Chem (1)], opening new avenues for peptide drug development. Further enhancements with multiple triazole substitutions yielded conjugates with greater stability and receptor affinity (Fig. 1). Radiolabeled multi-triazolo-peptidomimetics showed up to 4-fold higher tumor uptake versus the all-amide reference compound [Grob et al., 2020. J Med Chem (2)]. Importantly, a linear peptidomimetic with three triazole insertions retained full biological activity.
Radiolabeled exendin-4 binds with high affinity to the glucagon-like peptide-1 receptor (GLP-1R), which is highly expressed on the pancreatic β-cells and plays an important role in glucose metabolism and β-cell-derived diseases including diabetes or insulinoma, a rare type of neuroendocrine tumor. Our study developed exendin-4 derivatives by introducing albumin binding moiety [Kaeppeli et al., 2019. Mol Pharm] for reduced kidney retention and improved targeting of GLP1R-positive cells. Furthermore, we have shown that 18F-labeled silicon containing exendin-4 peptide [Dialer et al., 2018. EJNMMI Radiopharm Chem] or introduction of desferrioxamine (DFO) chelating system in combination with exendin-4 [Kaeppeli et al., 2019 EJNMMI Radiopharm Chem] is a feasible option for clinical imaging of insulinomas.
Targeting overexpressed G-protein coupled receptors (GPCRs) with bivalent radiolabeled ligands is a promising strategy for cancer imaging and therapy. We used experimental and computational approaches to study oligoproline-based homobivalent ligands targeting either the gastrin-releasing peptide receptor (GRPR) or somatostatin receptor 2A (SSTR2A). A 20 Å distance between recognition motifs yielded optimal cellular uptake for both receptors. Interestingly, a 10 Å spacing also enhanced uptake, indicating that both GPCR homodimer targeting and statistical rebinding contribute to the cellular uptake [Dobitz et al., 2020. Bioconjug Chem]. Moreover, rigid oligoproline bivalent GRPR ligands stabilized receptor dimers independently of their physiological relevance, offering valuable insights into GPCR dimerization and expanding the utility of bivalent ligands in drug design [Romantini et al., 2021. Proc Natl Acad Sci U S A].
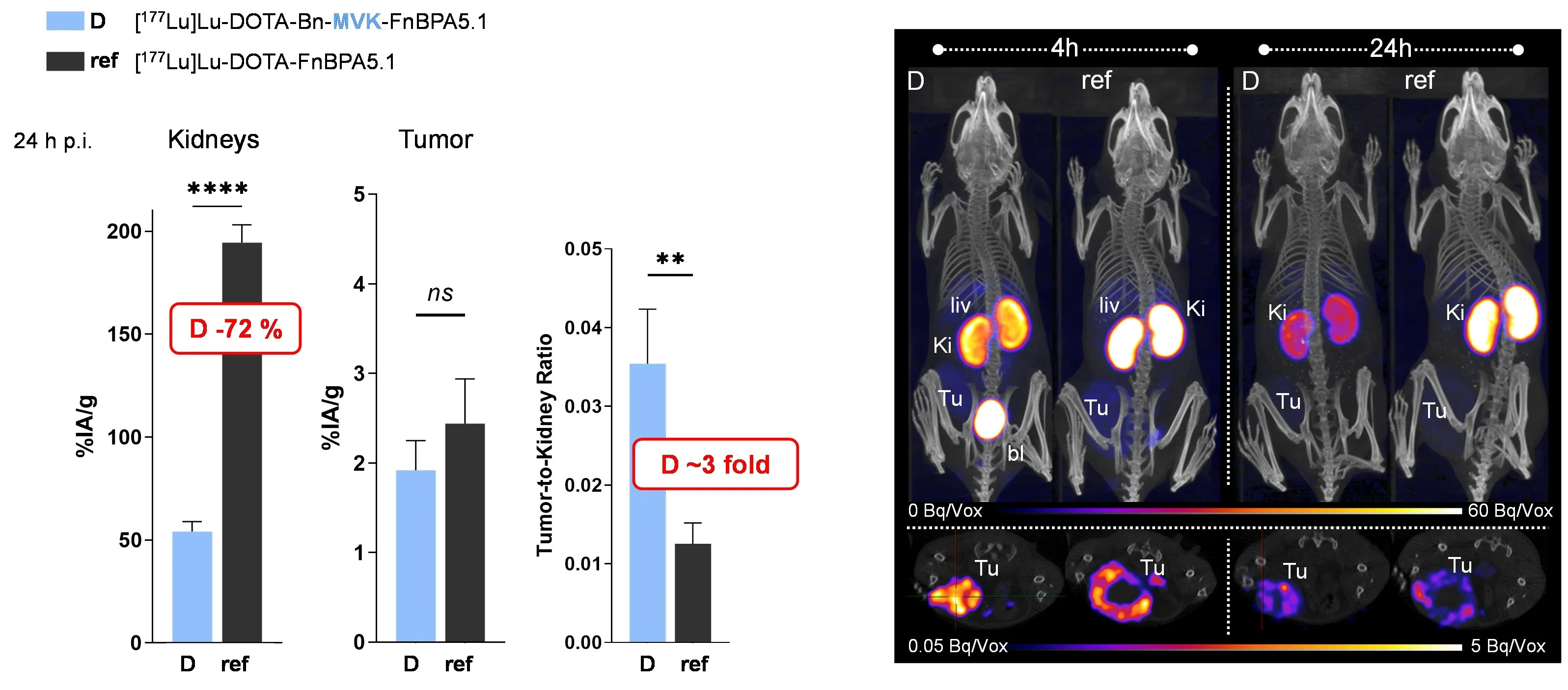
A major challenge in RLT is kidney clearance and reuptake, which can cause high renal accumulation and potential nephrotoxicity. Thus, various strategies have been developed to reduce kidney retention and improve the therapeutic efficacy of radioligands [de Roode et al., 2024. Pharmaceuticals]. Recently, we developed fibronectin-binding peptides (FnBPs) with dual MVK-based cleavable linkers. This dual-linker strategy significantly reduced renal retention and improved tumor-to-kidney ratios compared to derivatives featuring a single MVK unit [Valpreda et al., 2022. Bioorg Med Chem] (Fig. 2). Furthermore, we showed reduced kidney uptake of radiolabeled exendin-4 by using variants of the renally cleavable linker MVK in GLP-1R tumor-bearing mice [Trachsel et al., 2023. EJNMMI Radiopharm Chem]. This strategy is an important step towards the clinical translation and might as well be adopted for other radiopharmaceuticals suffering from persistent renal retention of radioactivity.
Dual MVK cleavable linkers effectively reduce renal retention of radiolabeled fibronectin-binding peptides. Left panel; Biodistribution of 177Lu-labeled DOTA-Bn-MVK(hex-FnBPA5.1) (D) in comparison to 177Lu-DOTA-FnBPA5.1 (ref) in 67NR breast tumor bearing mice at 24 h post injection (p.i.). Right panel; Representative images of in vivo SPECT/CT scans of 67NR mice obtained at 4 and 24 h p.i.
The high lipophilicity of the therapeutic radioligands leads to slow clearance from the bloodstream and high accumulation in non-target healthy tissues. Thus, we are focusing on increasing hydrophilicity within the linker region, with the goal of improving pharmacokinetic properties while preserving biological activity. Currently, we are conducting a structure-guided design and validation study of radioligands targeting G-protein coupled receptor 4 (GPR4), aiming to develop a new class of hydrophilic agents for cancer theranostics.
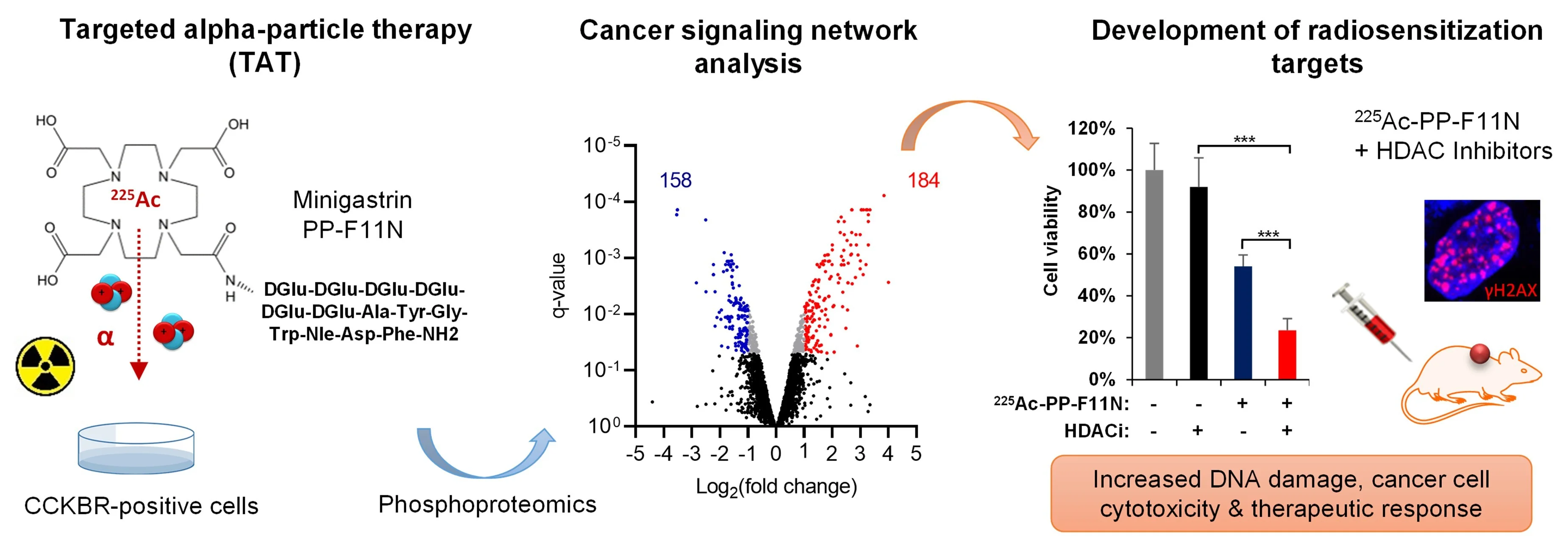
Targeted radionuclide therapy (TRT) delivers cytotoxic radiation to cancer lesions but is often limited by cancer radioresistance, insufficient radioligand delivery, or toxicity to healthy tissues. Strategies to enhance radiosensitization or tumor-specific radiopharmaceutical uptake hold promise for improving efficacy and safety. Our study focuses on understanding of the molecular mechanisms of cancer responses via signaling networks to radiolabeled peptides or antibodies, and biology of targeted receptors to develop more effective treatments. Using phosphoproteomics and drug library screens we investigate radiobiology across different particle radiations to identify radiosensitization targets for combinatorial therapies. Our phosphoproteomics studies in medullary thyroid cancer (MTC) models revealed key signaling alterations following treatment with the minigastrin analogue PP-F11N radiolabeled with lutetium-177 (β¯-particles) [Grzmil et al., 2022. J Hematol Oncol] and actinium-225 (α-particles) [Qin et al., 2023. J Nucl Med]. More recently, phosphoproteomics of anti-CD30 radioimmunotherapy (RIT) using terbium-161 (conversion and Auger electrons / β¯-particles) in a lymphoma animal model uncovered activated signaling networks, providing mechanistic insights into its superior therapeutic effects compared to lutetium-177 RIT [Rioja-Blanco et al., 2025. J Nucl Med]. These studies also identified potential radiosensitizing targets and evaluated small-molecule inhibitors to enhance the efficacy of radioligand therapy (RLT) in MTC models (Fig. 3).
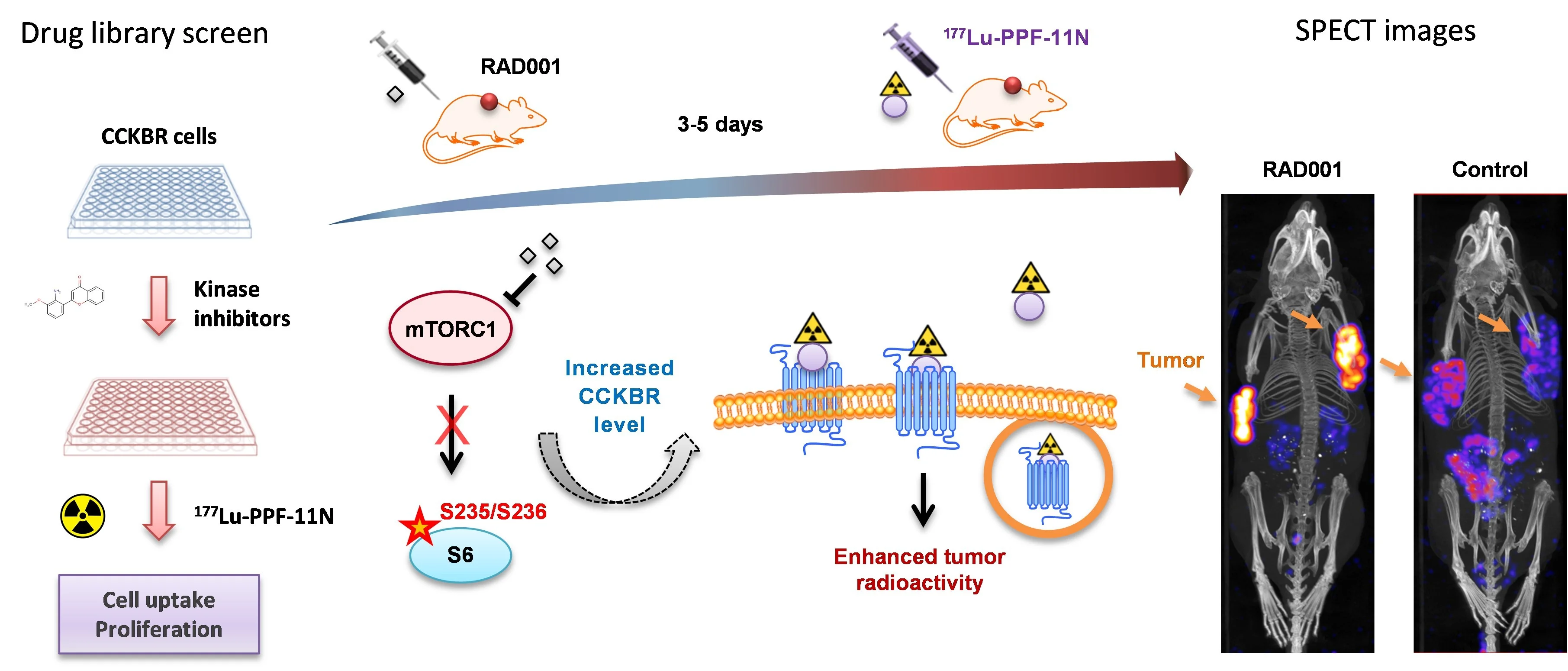
A high tumor-to-healthy-tissue uptake ratio of radiolabeled ligands is an essential prerequisite for safe and effective RLT. Our drug library screens revealed that pharmacological inhibition of mammalian target of rapamycin complex 1 (mTORC1) signaling augments tumor-specific uptake of [177Lu]Lu-PP-F11N [Grzmil et al., 2020. Theranostics], and significantly extended median survival time of treated mice, as compared to the monotherapy [Grzmil et al., 2021 Pharmaceutics], providing a clinically feasible strategy to enhance RLT efficacy and nuclear imaging (Fig. 4).
Drug library screen for identification of efficacious combinatory treatments. Pharmacological inhibition of mTORC1 by everolimus (RAD001) increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue 177Lu-PP-F11N in preclinical MTC models. Adapted from Grzmil et al., 2020. Theranostics.
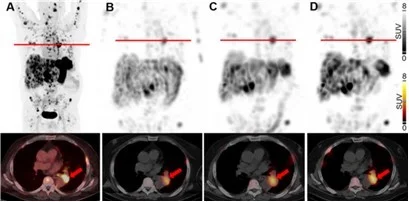
Remarkably, first single patient therapy at University Hospital Basel validated our strategy for enhanced uptake of radiolabeled minigastrin in everolimus-treated MTC patient (Fig. 5) [Rottenburger et al., 2025. J Nucl Med]. Funded by the Swiss National Science Foundation (SNF), we are currently expanding our radiobiology study. This research explores how the location of therapeutic targets - such as internalized or membrane-bound proteins on cancer cells, proteins on surrounding microenvironmental cells (e.g., fibroblast activation protein (FAP) on cancer-associated fibroblasts (CAF)), or extracellular proteins (e.g., fibronectin), and the type of radioactive emitter used (β-, α, or Auger electrons) influence the effectiveness of targeted radiotherapies at the cellular and molecular levels.
Maximum-intensity projections and transaxial slices of 68Ga-PP-F11N PET/CT (A) and posttherapeutic, quantitative SPECT/CT 72 h after the 1st (B), 2nd (C) and 3rd (D) RLT cycle of 177Lu-PP-F11N, showing metastatic tracer uptake, increasing with the number of RLT cycles. Two weeks prior the 3rd RLT cycle, the patient received additionally 10 mg everolimus daily, resulting in a further increase of tumor uptake. Red arrows indicate a mediastinal tumor lesion. Adapted from Rottenburger et al., 2025. J Nucl Med.
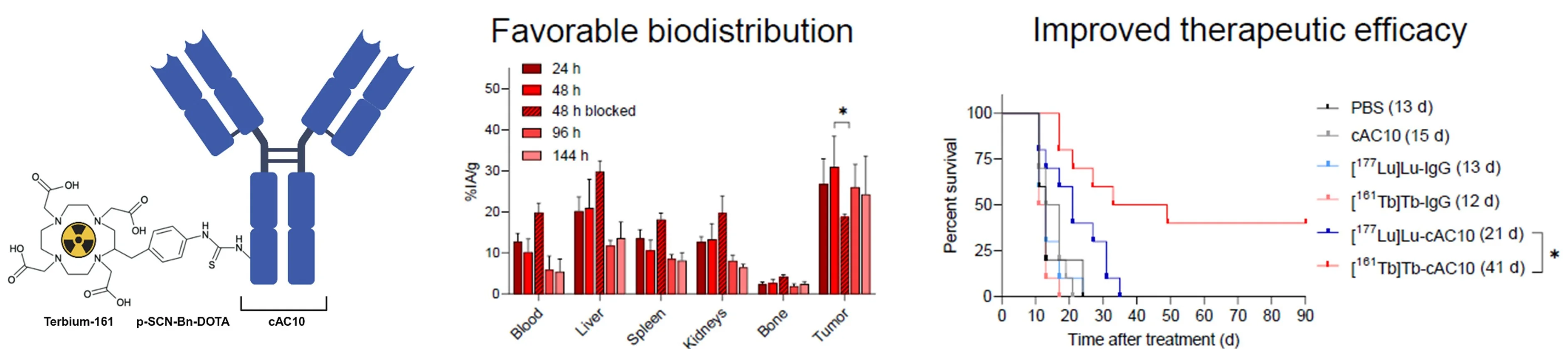
One of the key focuses of our group is the development of RIT by exploiting the unique radiobiological properties of conversion electron and Auger electron (CE/AE) emitters. Due to their ultra-short range in tissue and relatively high linear energy transfer, they can eliminate single tumor cells and small clusters, particularly relevant in disseminated blood cancers or highly tumorigenic cancer stem cells (CSCs). Our recent study revealed the therapeutic potential of terbium-161, a novel radionuclide emitting both β¯-particles and CE/AE, conjugated to the anti-CD30 antibody cAC10 for the treatment of CD30-positive T-cell lymphomas, which have only limited therapeutic options [Rioja-Blanco et al., 2025. J Nucl Med]. Compared to the standard lutetium-177, terbium-161 showed a favorable biodistribution and significantly improved survival in a xenograft mouse model of the disease (Fig. 6).
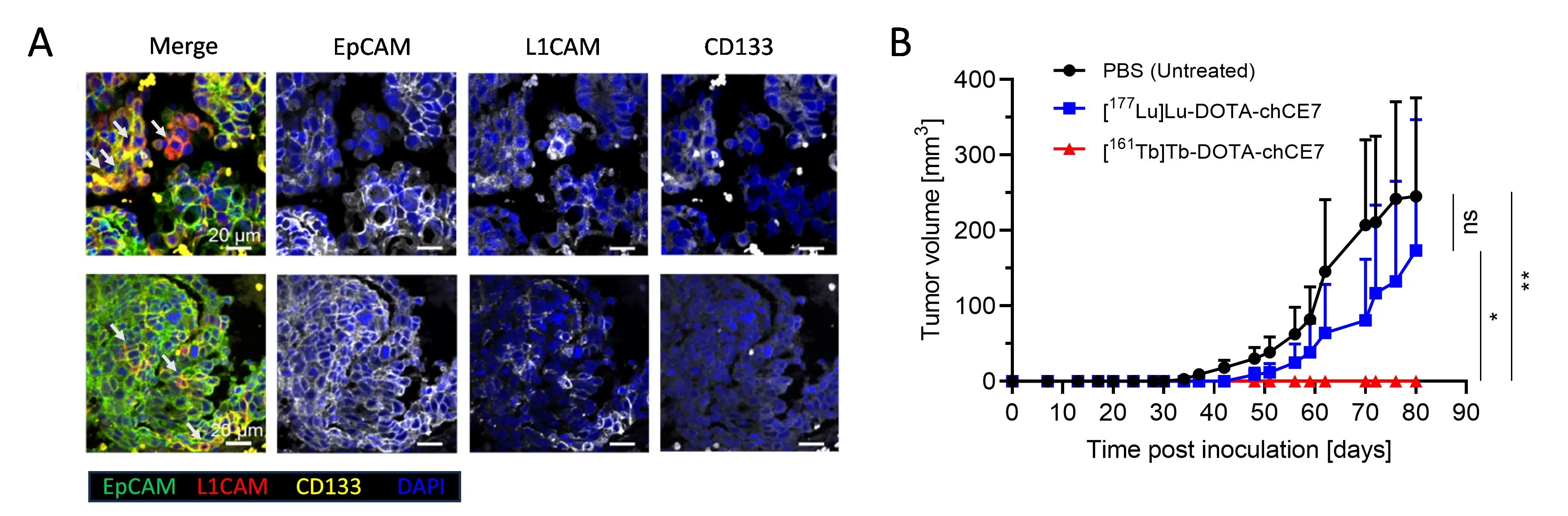
Furthermore, our preclinical research established terbium-161 RIT as a novel therapeutic modality against CSCs, which play a key role in tumor relapse, metastasis, and therapy resistance. Targeting CSC-associated biomarker L1 cell adhesion molecule (L1CAM) using terbium-161 anti-L1CAM RIT eliminated CSCs more efficiently than lutetium-177 anti-L1CAM RIT in both ovarian cancer in vitro and in vivo models, highlighting terbium-161's enhanced cytotoxicity against radioresistant tumors and its promising therapeutic potential [Todorov et al., 2025. J Nucl Med] (Fig 7).
Collectively, these studies represent the first preclinical evaluation of terbium-161 RIT in lymphoma and ovarian cancer models highlighting its therapeutic potential in other hematological and disseminated malignancies. Our current efforts focus on testing terbium-161 in more clinically relevant mouse models of disseminated and metastatic disease.
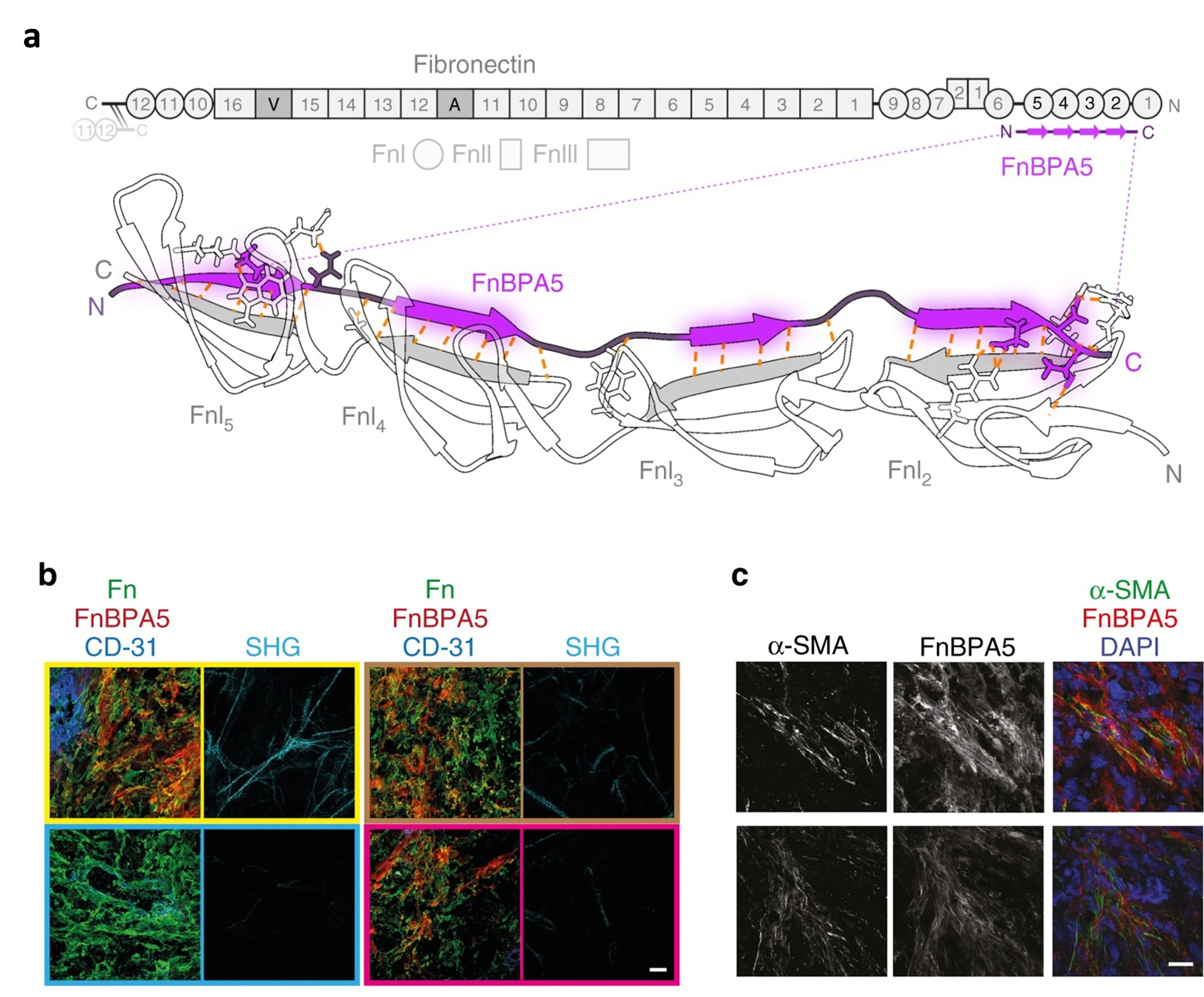
The tumor microenvironment (TME) has emerged as an attractive diagnostic or therapeutic target. The TME consists of malignant cells and a diverse population of non-malignant components, including immune cells, cancer-associated fibroblasts (CAFs), and endothelial cells (ECs), all embedded within a complex extracellular matrix (ECM) composed of fibronectin, collagen, elastin, and laminin. Remodeling of the ECM is a hallmark of tumor progression, yet the mechanisms by which interactions of cancer cells and ECM drive these changes is not fully understood. Previously, we introduced a novel nanoprobe to assess mechanical strain in fibronectin (Fn) fibers in situ, utilizing the bacterial fibronectin-binding peptide FnBPA5 [Arnoldini et al., 2017. Nat Commun] (Fig. 8).
Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. (a) Schematic representation of the multidomain protein fibronectin with epitope for FnBPA5 which binds to modules FnI2–5 via an antiparallel beta-zipper. (b) Stains of FnBPA5 (red), fibronectin (green), CD-31 (blue), and mature collagen fibers visualized by SHG (cyan) as well as the representative z-projection images (c) for α-SMA and FnBPA5 merged with nuclei staining (DAPI) in tumor cryosection from prostate cancer (PC-3) xenografts. Scale bar, 20 μm. Adapted from Arnoldini et al., 2017. Nat Commun.
This high-affinity peptide enables the specific targeting and visualization of relaxed fibronectin in tissues, expanding our understanding of its role in the pathophysiology of cancer and other diseases. In a recent study, we validated relaxed fibronectin as a promising imaging biomarker for endometriosis, leading to the development of the diagnostic radiotracer [¹¹¹In]In-FnBPA5 [Trachsel et al., 2024, EJNMMI Res]. Currently, our research focuses on targeting proteins expressed by both malignant and stromal cells, including G-protein coupled receptors (GPCRs) and fibroblast activation protein (FAP), as well as ECM components such as Tenascin-C (TNC) and relaxed fibronectin. We employ radiolabeled small molecules, peptides, and nanobodies and develop clinically relevant in vitro models including heterogeneous 2D and 3D cultures as well as in vivo syngeneic and orthotopic mouse models to evaluate these radiotracers.
Small benign insulinomas are often challenging to localize due to their size and subtle presentation, which can complicate the planning and execution of surgical interventions. In a previous collaborative study, we evaluated the diagnostic performance of single-photon emission computed tomography combined with computed tomography (SPECT/CT) using a glucagon-like peptide-1 (GLP-1) receptor avid radiotracer for insulinoma detection [Christ et al., 2013. Lancet Diabetes Endocrinol]. The clinical data demonstrated that [¹¹¹In]In-DTPA-exendin-4 SPECT/CT offers a valuable second-line imaging modality for patients with inconclusive or negative findings on conventional imaging techniques such as CT or MRI.
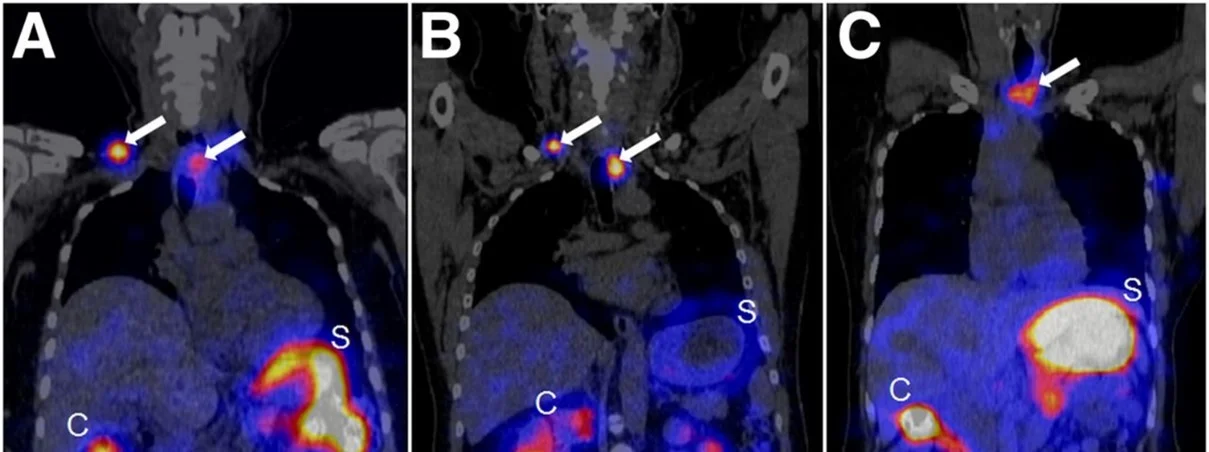
The treatment of patients with advanced medullary thyroid carcinoma (MTC) remains another significant clinical challenge. The cholecystokinin B receptor (CCKBR) has emerged as a promising target for MTC therapy using radiolabeled minigastrin analogs. However, kidney toxicity limits their therapeutic application. In the collaborative study with University Hospital Basel theragnostic suitability of developed by our group radiolabeled minigastrin analog [177Lu]Lu-PP-F11N was evaluated in MTC patients. The first clinical application of [177Lu]Lu-PP-F11N revealed efficient intratumor accumulation by single photon emission computed tomography (SPECT) in MTC patients [Sauter et al., 2018. J Nucl Med]. More recently, high tumor accumulation of metabolically stable radiolabeled minigastrin [177Lu]Lu-PP-F11N was demonstrated in MTC patients with low adverse reactions [Rottenburger et al., 2020. J Nucl Med] (Fig. 9).
Importantly, the compound exhibited a favorable biodistribution profile with low radiation doses to the kidneys and bone marrow, although the stomach was identified as the dose-limiting organ. Currently, further clinical investigations of [¹⁷⁷Lu]-PP-F11N are underway, including combination with other treatment modalities, to improve tumor-specific uptake and therapeutic efficacy in MTC patients.
Publications Pharmacology Group
Group members
- Rottenburger C, Freitag MT, Omrane MA, Hentschel M, Behe M, Grzmil M, Meyer PT, Wild D. The "Two-Step Boost" for [177Lu]Lu-PP-F11N Therapy: Optimization of Tumor Uptake by Premedication. J Nucl Med. 2025 Sep 11:jnumed.125.270588. doi: 10.2967/jnumed.125.270588.
- Rioja-Blanco E, Banz Y, Schlapbach C, Novak U, Chiorazzo T, Bertschi NL, Bernhardt P, Grundler PV, van der Meulen NP, Grzmil M, Schibli R, Behe M. 161Tb Radioimmunotherapy as a Treatment for CD30-Positive Lymphomas. J Nucl Med. 2025 Jun 2;66(6):909-915. doi: 10.2967/jnumed.124.268805.
- Todorov TZ, Coelho R, Dellea S, Jacob F, Heinzelmann-Schwarz V, Grundler PV, van der Meulen NP, Béhé MP, Schibli R, Grünberg J. 161Tb-Based Anti-L1CAM Radioimmunotherapy Shows Superior Efficacy in Eliminating Ovarian Cancer Stem Cells Compared with 177Lu in Preclinical Models of Ovarian Cancer. J Nucl Med. 2025 Jun 5:jnumed.124.269078. doi: 10.2967/jnumed.124.269078.
- Trachsel B, Imobersteg S, Valpreda G, Singer G, Grabherr R, Ormos M, Burger IA, Kubik-Huch RA, Schibli R, Vogel V, Béhé M. Relaxed fibronectin: a potential novel target for imaging endometriotic lesions. EJNMMI Res. 2024 Feb 10;14(1):17. doi: 10.1186/s13550-024-01070-0.
- de Roode KE, Joosten L, Behe M. Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals (Basel). 2024 Feb 16;17(2):256. doi: 10.3390/ph17020256.
- Qin Y, Imobersteg S, Frank S, Blanc A, Chiorazzo T, Berger P, Schibli R, Béhé MP, Grzmil M. Signaling Network Response to α-Particle-Targeted Therapy with the 225Ac-Labeled Minigastrin Analog 225Ac-PP-F11N Reveals the Radiosensitizing Potential of Histone Deacetylase Inhibitors. J Nucl Med. 2023 Jun;64(6):873-879. doi: 10.2967/jnumed.122.264597.
- Trachsel B, Valpreda G, Lutz A, Schibli R, Mu L, Béhé M. Reducing kidney uptake of radiolabelled exendin-4 using variants of the renally cleavable linker MVK. EJNMMI Radiopharm Chem. 2023 Sep 4;8(1):21. doi: 10.1186/s41181-023-00206-2.
- Grzmil M, Boersema P, Sharma A, Blanc A, Imobersteg S, Pruschy M, Picotti P, Schibli R, Behe M. Comparative analysis of cancer cell responses to targeted radionuclide therapy (TRT) and external beam radiotherapy (EBRT). J Hematol Oncol. 2022 Aug 31;15(1):123. doi: 10.1186/s13045-022-01343-y.
- Valpreda G, Trachsel B, Vogel V, Schibli R, Mu L, Behe M. Dual MVK cleavable linkers effectively reduce renal retention of 111In-fibronectin-binding peptides. Bioorg Med Chem. 2022 Nov 1;73:117040. doi: 10.1016/j.bmc.2022.117040.
- Grzmil M, Imobersteg S, Blanc A, Frank S, Schibli R, Béhé MP. Therapeutic Response of CCKBR-Positive Tumors to Combinatory Treatment with Everolimus and the Radiolabeled Minigastrin Analogue [177Lu]Lu-PP-F11N. Pharmaceutics. 2021 Dec 15;13(12):2156. doi: 10.3390/pharmaceutics13122156.
- Romantini N, Alam S, Dobitz S, Spillmann M, De Foresta M, Schibli R, Schertler GFX, Wennemers H, Deupi X, Behe M, Berger P. Exploring the signaling space of a GPCR using bivalent ligands with a rigid oligoproline backbone. Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2108776118. doi: 10.1073/pnas.2108776118.
- Grzmil M, Qin Y, Schleuniger C, Frank S, Imobersteg S, Blanc A, Spillmann M, Berger P, Schibli R, Behe M. Pharmacological inhibition of mTORC1 increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue [177Lu]Lu-PP-F11N. Theranostics. 2020 Aug 29;10(24):10861-10873. doi: 10.7150/thno.45440.
- Rottenburger C, Nicolas GP, McDougall L, Kaul F, Cachovan M, Vija AH, Schibli R, Geistlich S, Schumann A, Rau T, Glatz K, Behe M, Christ ER, Wild D. Cholecystokinin 2 Receptor Agonist 177Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma: Results of the Lumed Phase 0a Study. J Nucl Med. 2020 Apr;61(4):520-526. doi: 10.2967/jnumed.119.233031.
- Grob NM, Häussinger D, Deupi X, Schibli R, Behe M, Mindt TL. Triazolo-Peptidomimetics: Novel Radiolabeled Minigastrin Analogs for Improved Tumor Targeting. J Med Chem. 2020 May 14;63(9):4484-4495. doi: 10.1021/acs.jmedchem.9b01936.
- Grob NM, Schmid S, Schibli R, Behe M, Mindt TL. Design of Radiolabeled Analogs of Minigastrin by Multiple Amide-to-Triazole Substitutions. J Med Chem. 2020 May 14;63(9):4496-4505. doi: 10.1021/acs.jmedchem.9b01937.
- Dobitz S, Wilhelm P, Romantini N, De Foresta M, Walther C, Ritler A, Schibli R, Berger P, Deupi X, Béhé M, Wennemers H. Distance-Dependent Cellular Uptake of Oligoproline-Based Homobivalent Ligands Targeting GPCRs-An Experimental and Computational Analysis. Bioconjug Chem. 2020 Oct 21;31(10):2431-2438. doi: 10.1021/acs.bioconjchem.0c00484.
- Ritler A, Shoshan MS, Deupi X, Wilhelm P, Schibli R, Wennemers H, Béhé M. Elucidating the Structure-Activity Relationship of the Pentaglutamic Acid Sequence of Minigastrin with Cholecystokinin Receptor Subtype 2. Bioconjug Chem. 2019 Mar 20;30(3):657-666. doi: 10.1021/acs.bioconjchem.8b00849.
- Sauter AW, Mansi R, Hassiepen U, Muller L, Panigada T, Wiehr S, Wild AM, Geistlich S, Béhé M, Rottenburger C, Wild D, Fani M. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J Nucl Med. 2019 Mar;60(3):393-399. doi: 10.2967/jnumed.118.207845.
- Kaeppeli SAM, Schibli R, Mindt TL, Behe M. Comparison of desferrioxamine and NODAGA for the gallium-68 labeling of exendin-4. EJNMMI Radiopharm Chem. 2019 May 16;4(1):9. doi: 10.1186/s41181-019-0060-9.
- Kaeppeli SAM, Jodal A, Gotthardt M, Schibli R, Béhé M. Exendin-4 Derivatives with an Albumin-Binding Moiety Show Decreased Renal Retention and Improved GLP-1 Receptor Targeting. Mol Pharm. 2019 Sep 3;16(9):3760-3769. doi: 10.1021/acs.molpharmaceut.9b00271.
- Grzmil, M., Meisel, A., Behe, M. and Schibli, R. (2019) An Overview of Targeted Radiotherapy. In: Lewis, J.S., Windhorst, A.D. and Zeglis, B.M., Eds., Radiopharmaceutical Chemistry, Springer, Berlin, 91. https://doi.org/10.1007/978-3-319-98947-1_5
- Dialer LO, Jodal A, Schibli R, Ametamey SM, Béhé M. Radiosynthesis and evaluation of an 18F-labeled silicon containing exendin-4 peptide as a PET probe for imaging insulinoma. EJNMMI Radiopharm Chem. 2018;3(1):1. doi: 10.1186/s41181-017-0036-6.
- Arnoldini S, Moscaroli A, Chabria M, Hilbert M, Hertig S, Schibli R, Béhé M, Vogel V. Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat Commun. 2017 Nov 27;8(1):1793. doi: 10.1038/s41467-017-01846-0.
- Christ E, Wild D, Ederer S, Béhé M, Nicolas G, Caplin ME, Brändle M, Clerici T, Fischli S, Stettler C, Ell PJ, Seufert J, Gloor B, Perren A, Reubi JC, Forrer F. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013 Oct;1(2):115-22. doi: 10.1016/S2213-8587(13)70049-4.