Unraveling degradation processes in a bipolar membrane CO2 electrolyzer by time-resolved X-ray tomographic microscopy
Employing a bipolar ion conducting membrane (BPM) in forward bias is a convenient solution for the biggest issues in the more common anion exchange membrane (AEM) CO2 co-electrolysis: the degradation of the performance caused by carbonate salt precipitation at the cathode and the decrease of net CO2 conversion caused by the crossover of this molecule from cathode to anode also requiring energy for downstream gas separation. However, the performance and stability of this device remain largely insufficient when using such a BPM configuration. To understand the reasons for this, we performed time-resolved X-ray tomographic microscopy of an operating BPM CO2 electrolyzer. The imaging method reveals partly unexpected degradation processes that result in design recommendations for improvement.
CO2-electrolyzers are often operated using anion exchange membranes (AEMs) that provide the quasi-alkaline reaction conditions favorable for the CO2 reduction (CO2R) at the cathode. However, these alkaline conditions also entail the formation of (bi)carbonate anions that, in combination with the cations stemming from the hydroxide-based solutions (e.g., KOH) fed at the anode, form salt precipitates that clog the cathode and cause cell failure. On top of this, the AEM transports these (bi)carbonte species from cathode to anode, where they get re-oxidized to carbon dioxide causing the undesirable CO2 crossover. These issues can be alleviated by using a forward bias bipolar membrane (FB-BPM) in which the cathodic AEM is pressed against a cation proton exchange membrane (PEM) at the anode, eliminating the need for hydroxide solutions at the anode and as such the formation of (bi)carbonte salt(s). Moreover, these (bi)carbonate ions are expected to recombine with the protons in the PEM at the AEM-PEM interface, forming CO2 and water and decreasing the CO2-crossover issue.
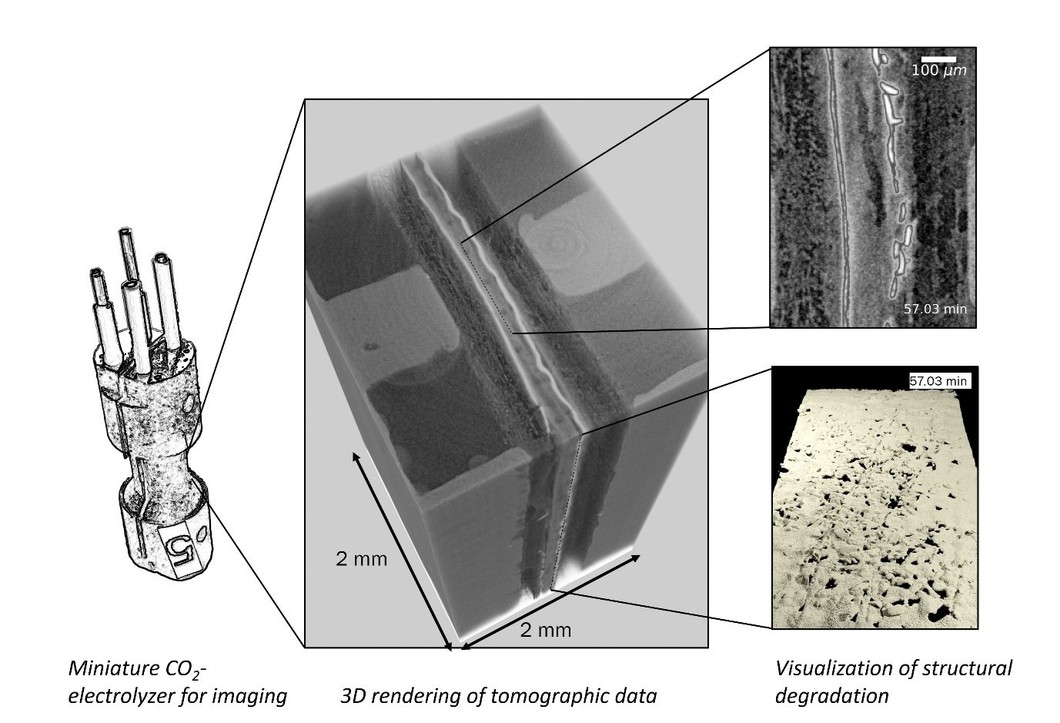
Despite these premises, the performance of FB-BPM CO2-electrolyzers is highly unstable for reasons that remain undetermined. To shed light on this instability, we operated a miniature CO2-electrolyzer at the TOMCAT beamline of the SLS and used time-resolved X-ray tomography to track structural dynamics in the cell. Using advanced image processing methods including custom 4D machine learning segmentation, we visualized and quantified damages in the membrane and anode CL. This unveiled that at high current densities the PEM and AEM interfaces delaminate due to excessive CO2 accumulation. More surprisingly, the anode catalyst layer (CL) quickly disintegrated during operation even at significantly lower currents.
We compared our results to a CO2 transport model from which we hypothesize that gaseous CO2 is the cause of the observed structural damages. More precisely, this CO2 does not remain static at the center of the BPM (i.e., within the PEM-AEM interface), but migrates to the anode through diffusion and accumulates within the gas phase at the interface between the PEM and the anode CL. After sufficient CO2 accumulation and pressure build-up, small holes irreversibly break along the entire active area of the anode CL.
These results indicate that the modification of the BPM (e.g., through changes in the composition and/or topology of the layers and junction) in a way to enhance CO2 back-diffusion towards the cathode should not only alleviate this degradation, but also improve the net CO2-utilization. Thus, our ongoing research already focus on such tailoring of the BPM properties.