Insights into radical induced degradation of anion exchange membrane constituents
Electrochemical energy conversion devices, such as fuel cells and electrolyzers, using an anion exchange membrane (AEM) operating in the alkaline regime offer the prospect of the use of non-noble metal electrocatalysts and lower-cost cell construction materials. The wide-spread application of electrochemical cells with AEMs has been largely limited by the low chemical stability of the material. AEM degradation is triggered by i) nucleophilic attack by OH−, and ii) by reaction with free radicals formed during cell operation. Whereas the alkaline stability of AEMs has been greatly increased over the last 10 years, the understanding of mechanisms of radical induced degradation is limited. In this study, we have addressed this topic for the first time.
Electrochemical cells using membrane electrolytes and a zero-gap configuration have a low ohmic resistance, thus enable operation at high current density (in the range of A/cm2), and allow a compact stack design with high power density. Proton exchange membranes (PEMs) have been used successfully in fuel cells and water electrolyzers for decades and reached a high level of technical maturity. The acidic environment in the cell, however, necessitates the use of noble metal electrocatalysts, for reasons of activity and stability, for the hydrogen and oxygen half-cell reactions. Alkaline conditions allow the use of non-noble metal catalysts and cheaper cell build materials (such as nickel instead of titanium), thus reducing cost and the use of critical raw materials. AEMs operating under alkaline conditions are susceptible to nucleophilic attack by OH−, yet significant improvements have been made over the past ~10 years. Today, several suppliers (e.g., Dioxide Materials, Versogen, Orion Polymer, Xergy, Ionomr) offer AEMs for fuel cells and electrolysis cells. It has been known for a long time that the chemical durability of PEMs is limited by radical induced degradation. The radical induced aging of AEMs, however, has received little attention. The increase in alkaline stability of AEMs increases the importance of studying and improving stability of AEMs against radical induced polymer breakdown.
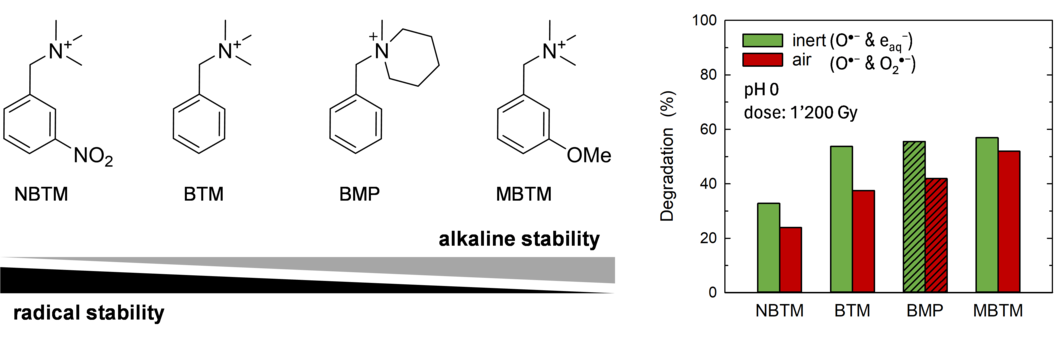
The presence of radicals in AEM fuel cells during operation has been confirmed experimentally and reported in the literature. Radicals form as a result of the interaction of H2, O2 and the electrocatalyst. The type of prevalent radical species depends strongly on the local pH in the cells. The pKa of radicals are 4.8 for HOO·, 9.1 for H·, and 11.9 for HO·. This means that the dominant radicals at alkaline pH of >12 are O2·−, eaq−, and O·−. We studied the reaction of radicals in an aqueous solution with selected aromatic quaternary ammonium compounds, mimicking different AEM exchange groups, namely benzyl-trimethylammonium (BTM), N-benzyl-N-methyl-piperidinium (BMP), 3-methoxy-benzyltrimethylammonium (MBTM), and 3-nitro-benzyltrimethylammonium (NBTM). We used pulse radiolysis at ETH Zürich with time-resolved transient spectroscopy to study kinetics of radical reactions, and performed degradation studies where radicals are produced continuously at low dose rate using a 60Co gamma source at PSI.
Pulse radiolysis studies indicate that that the reaction of O·− with the benzyl-type quaternary ammonium (QA) compounds results in the formation of mainly reducing but also oxidizing species with a lifetime on the order of milliseconds. Steady-state gamma-radiolysis experiments show that the electron density of the aromatic ring critically influences the behavior of the compound. The presence of an electron-withdrawing group decreases alkaline stability by increasing the overall electrophilic character of the aromatic compound, thus rendering it more susceptible to nucleophilic attack by OH−. Concomitantly, stability against radical induced degradation is increased because an electron poor character destabilizes intermediate radical species that form following radical attack.
Therefore, a careful balance needs to be established between alkaline and radical durability. Under neutral and acidic conditions, HO· is the radical with the highest oxidative strength and readily reacts by addition to the aromatic ring. Yet at high pH, HO· is deprotonated, and the formed O·− reacts with the substrate mainly by hydrogen atom abstraction or by dealkylation through reduction. In analogy, at high pH hydrogen radicals, H·, will deprotonate to the highly reducing hydrated electron, eaq−, and will readily attack the QA groups, which results in fragmentation of the compound.
A key finding of the study is the understanding of the influence or ring electron density on the susceptibility to degradation of benzyl-type QA compounds: electron-donating constituents promote radical induced degradation, while electron withdrawing ones improve stability. Therefore, resistance against radical-induced degradation shows an opposite trend to alkaline stability, which is known to be decreased for electron-poor compounds due to their high reactivity towards the nucleophilic attack of OH−.