Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
Alkaline exchange membrane water electrolyzers (AEMWE) are a promising electrochemical energy conversion device to produce green hydrogen from intermittent renewable energies. The electrochemical reaction that suffers most from high overpotentials is the oxygen evolution reaction (OER). Transition metal oxide catalysts can exhibit relatively high OER activity and stability at high basicity (> pH 13), but their electrochemical properties degrade in near-neutral pH (7–10) environments. However, near-neutral pHs are highly relevant in practical applications. Using KOH electrolytes in AEMWE creates vulnerability to carbonation during operation, lowering the initially high pH level toward near-neutral pHs. Carbonation is a significant problem in recently established AEMWE systems using a carbonate membrane that operates as a co-electrolysis cell by replacing the cathodic hydrogen evolution reaction with carbon oxide reduction.
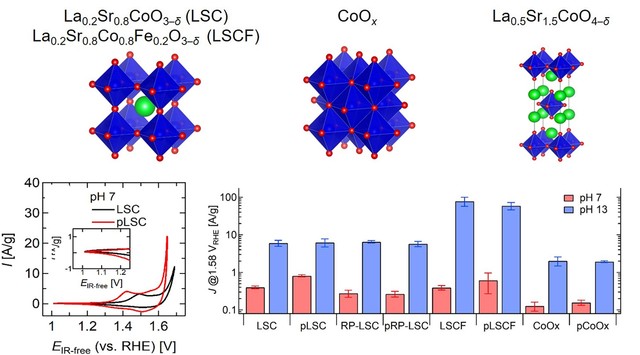
This study has investigated a dry phosphate ion treatment to functionalize nano-sized cobalt-based oxygen evolution reaction (OER) catalysts (La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, La0.5Sr1.5CoO4–δ, and CoOx) at near-neutral pHs. We proved that a dry treatment process using NaH2PO2·H2O as P source is effective for catalyst surface functionalization, especially for nano-sized catalysts. Electrochemical evaluations revealed that phosphate ion functionalized cobalt-based oxides, except for La0.5Sr1.5CoO4–δ, exhibited higher OER activity at neutral pH than the as-synthesized catalysts. The mechanism of deteriorated OER performance in La0.5Sr1.5CoO4–δ at neutral pH was successfully explained by surface-sensitive soft X-ray absorption and X-ray photoelectron spectroscopy measurements. Electrochemically inert SrO-based surface segregations likely inhibit the phosphate ion functionalization on the active cobalt layer of La0.5Sr1.5CoO4–δ and reduce OER activity, especially at near-neutral pHs. Differently, phosphate functionalization seems to be beneficial for CoOx, La0.2Sr0.8CoO3–δ, and La0.2Sr0.8Co0.8Fe0.2O3–δ, and particularly for the La0.2Sr0.8CoO3–δcatalyst. It is hypothesized that phosphate ion groups could assist the deprotonation step of the OER mechanism in electrolytes with low OH– concentration. In addition, we unveiled that P-treatment led to a reduced cobalt oxidation state in La0.2Sr0.8CoO3–δ, which could also boost its OER activity at neutral pH. Thus, it would be important in the future to decouple the effect of phosphate functionalization on the physicochemical properties of the catalysts from the phosphate ion role in the OER mechanism and kinetics. Additionally, optimization of the P functionalization process could result in a further improvement of OER activity at neutral pHs.