Importance of Identifying Key Experimental Parameters for the Li-ion Battery Performance Testing
The mass loading of Si-graphite electrodes is often considered as a parameter of secondary importance when testing their performance. However, if a sacrificial additive is present in the electrolyte, the electrode loading becomes the battery cycle-life-determining factor. A lower loading was obtained by keeping slurry preparation steps unchanged from binder to binder and resulted in a longer lifetime for some of the binders. When the final loading was kept constant instead, the performance became independent of the binder used.
The increasing market demand for high energy density batteries urge scientist to develop simple but feasible approaches to improve gravimetric and volumetric energy of commercial cells. This can be achieved either by increasing cells voltage or its capacity. One of the approaches is to increase the capacity of the anode, as capacity is cathodes is fundamentally more limited. The simplest method to do so is to mix graphite with capacity of 372 mAh/g, with a specific charge-enhancing component (an element or compound possessing a significantly higher capacity than graphite), such as silicon with capacity of 3572 mAh/g, i.e. the capacity about ten times higher than that of graphite.
However, with increasing fraction of silicon also renders cell cycling less stable, and different electrode engineering approaches are used to improve their cycling stability. One of the remedies often discussed in literature is functional binders, such as polyacrylic acid (PAA), sodium carboxymethyl cellulose (Na-CMC), and Na-alginate, because they are able to partially buffer Si volume expansion thanks to the formation of a cross-linking network and a strong interaction with Si particles. The second often used remedy is electrolyte additives, significantly improving the cycle life of silicon-containing electrodes, where most used is fluoroethylene carbonate (FEC).
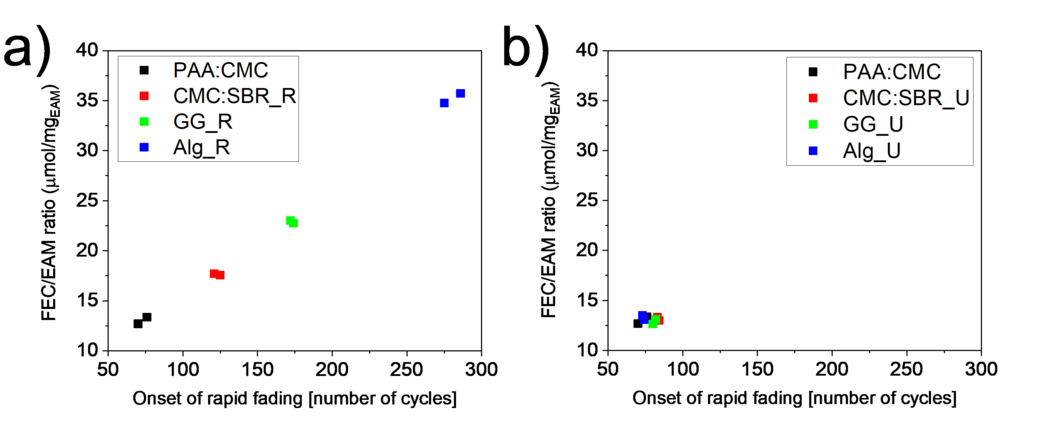
The long-term cycling stability of silicon-graphite electrodes in presence of additives indirectly depends on another important parameter: the electrode mass loading. Because the additives are often of the sacrificial character and are consumed during cycling, it has been proven that the lifetime of a cell, containing silicon electrodes, is linearly dependent on the ratio between the additive and active material loading. This means that larger amount of additive or lower mass loading will result in longer lifetime of the cell with the same amount of electrolyte. However, the electrode loading is a parameter often unreported in many scientific publications. Therefore, we performed a systematic study to show importance of these experimental parameters once and for all.
There is two approaches how to perform comparable experiments: either keep the procedure constant or keep the final testing set up constant, - in this case, either slurry recipe or mass loading of final electrode. Alternative approach would be to adjust electrolyte and additive amount to the loading, which case experiment reliability is less robust. Therefore, the goal of our study was to demonstrate the interrelation between electrolyte additive, electrode loading, and type of binder, and how these parameters influence the cycling stability of silicon-graphite electrodes.
The results of this study showed that in presence of constant amount of electrolyte additive, the loading plays a major role in determining the cell cycle-life, and therefore more attention has to be paid to this parameter in order to provide truthful results to the scientific and industrial communities. Electrodes with various mass loadings and different types of binder were fading after a number of cycles, the onset of which linearly correlated to additive/loading ratio and, contrary to the expectations, the battery cycle life did not depend on the type of binder. In the case when electrodes were prepared with different binder types but with very close values for active materials loading, the differences in cycle life were minor, which leads to conclusion that the FEC consumption per amount of active material per cycle is the same, independently of binder used.
This means that revisiting past results, where binder properties as performance-enhancing factors are considered using sacrificial additives, the careful scrutiny of experimental conditions and especially differences in electrode loading and electrolyte (additive) to active materials ratio should be done. Our results also show that in order to test effects of electrode composition changes (such as binder in our case), the same active material loading should be the guiding parameter and not the same electrode preparation conditions.