in the Laboratory of Environmental Chemistry (LUC)
Feinstaub ist gefährlicher als gedacht
Präziser Blick in feinste Partikel in der Luft zeigt, wie gesundheitsschädliche Verbindungen entstehen.
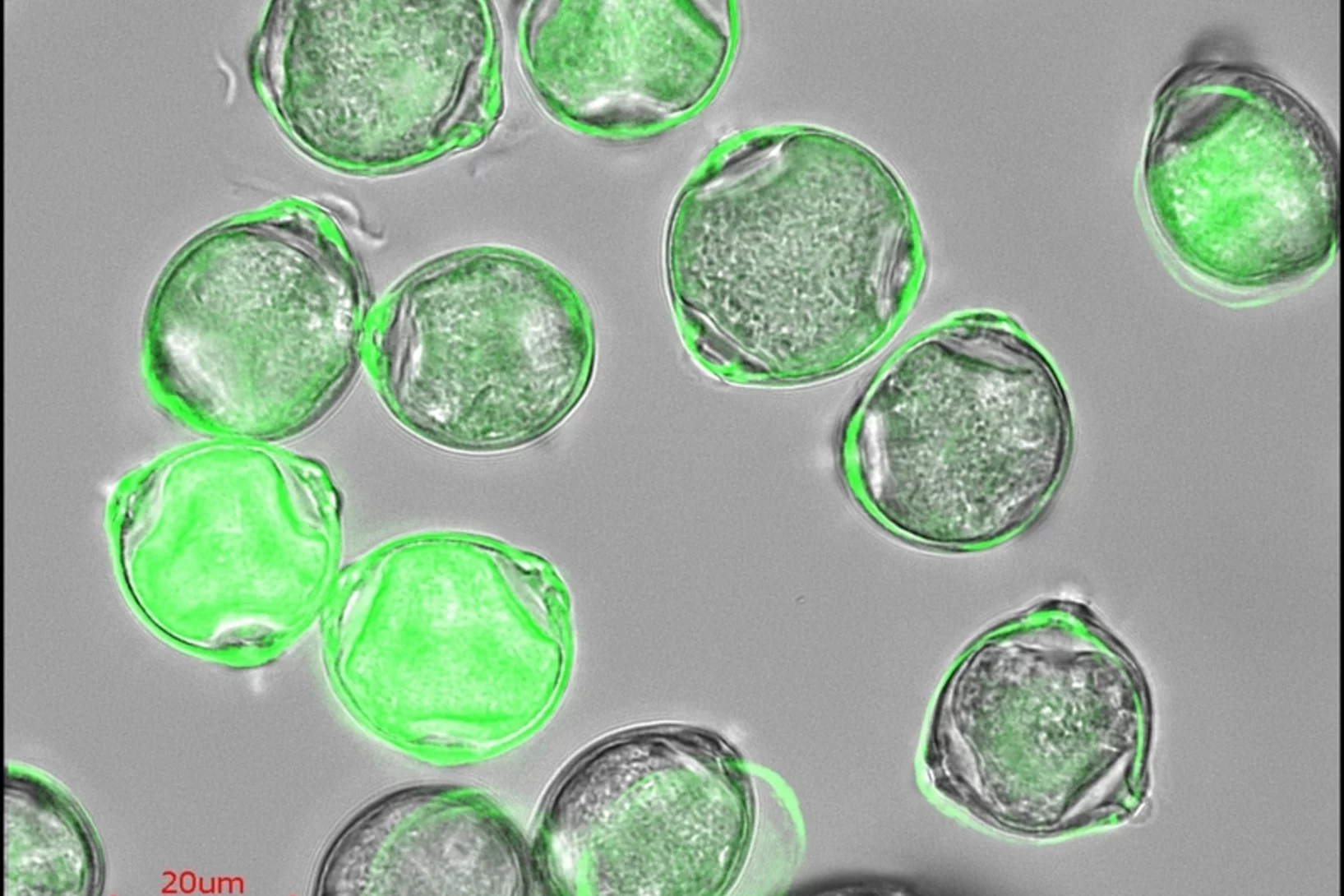
Chemically mapping ice forming particles
Scientists have just nucleated ice in an X-ray microscope for the first time and they created chemical maps of those responsible.
Warum die Kleine Eiszeit Mitte des 19. Jahrhunderts endete
In der ersten Hälfte des 19. Jahrhunderts führte eine Serie grosser Vulkanausbrüche in den Tropen zu einer vorübergehenden globalen Abkühlung des Erdklimas. Dass in der Endphase dieser sogenannten Kleinen Eiszeit die Alpengletscher wuchsen und anschliessend wieder zurückgingen, war ein natürlicher Prozess. Dies haben nun PSI-Forschende anhand von Eisbohrkernen nachgewiesen.
Light from the particle accelerator helps to understand ozone decomposition
PSI researchers have developed an experimental chamber in which they can recreate atmospheric processes and probe them with unprecedented precision, using X-ray light from the Swiss Light Source SLS. In the initial experiments, they have studied the production of bromine, which plays an essential role in the decomposition of ozone in the lower layers of the atmosphere. In the future, the new experiment chamber will also be available for use by researchers from other scientific fields.
Coexistence of Physisorbed and Solvated HCl at Warm Ice Surfaces
The interfacial ionization of strong acids is an essential factor of multiphase and heterogeneous chemistry in environmental science, cryospheric science, catalysis research and material science. Using Near Ambient Pressure X-ray Photoelectron (NAPP) spectroscopy, we directly detected a low surface coverage of adsorbed HCl at 253 K in both molecular and dissociated states and interpret the results as physisorbed molecular HCl at the outermost ice surface and dissociation occurring upon solvation deeper in the interfacial region. This study gives clear evidence for nonuniformity across the air−ice interface and questions the use of acid−base concepts in interfacial processes.
Ice-core evidence of earliest extensive copper metallurgy in the Andes 2700 years ago
Although copper (Cu) was essential for the wealth of pre- and post-colonial societies in the Andes, the onset of extensive Cu metallurgy in South America is still debated. Based on a 6500 year ice-core Cu record from Illimani glacier in Bolivia we provide the first complete history of large-scale Cu smelting activities in South America. Earliest anthropogenic Cu pollution was found during the Early Horizon period ~700-50 BC. We attribute the onset of intensified Cu smelting in South America to the activities of the central Andean Chiripa and Chavin cultures ~2700 years ago. This study provides for the first time substantial evidence for extensive Cu metallurgy already during these early cultures.
Organic Nitrate Contribution to New Particle Formation and Growth in Secondary Organic Aerosols from α-Pinene Ozonolysis
The chemical kinetics of organic nitrate production during new particle formation and growth of secondary organic aerosols (SOA) were investigated using the short-lived radioactive tracer 13N in flow-reactor studies of α-pinene oxidation with ozone. Direct and quantitative measurements of the nitrogen content indicate that organic nitrates accounted for ∼40% of SOA mass during initial particle formation, decreasing to ∼15% upon particle growth to the accumulation-mode size range (>100 nm). Experiments with OH scavengers and kinetic model results suggest that organic peroxy radicals formed by α-pinene reacting with secondary OH from ozonolysis are key intermediates in the organic nitrate formation process
Pb pollution from leaded gasoline in South America in the context of a 2000-year metallurgical history
Exploitation of the extensive polymetallic deposits of the Andean Altiplano in South America since precolonial times has caused substantial emissions of neurotoxic lead (Pb) into the atmosphere; however, its historical significance compared to recent Pb pollution from leaded gasoline is not yet resolved. We present a comprehensive Pb emission history for the last two millennia for South America, based on a continuous, high-resolution, ice core record from Illimani glacier. Illimani is the highest mountain of the eastern Bolivian Andes and is located at the northeastern margin of the Andean Altiplano.
Polychlorinated biphenyls in glaciers
We present a highly time-resolved historical record of polychlorinated biphenyls (PCBs) from an Alpine ice core (Fiescherhorn glacier, Switzerland). Introduced in the 1940s, PCBs were widely used industrial chemicals. Because of their persistence they are still found in the environment, long after their production phase-out. The Fiescherhorn ice core record covers the entire time period of industrial use of PCBs, that is, 1940?2002. The total concentration of six PCBs varies from 0.5 to 5 ng/L and reveals a temporal trend, with an 8-fold increase from the early 1940s to the peak value in the 1970s.
Ice-core based assessment of historical anthropogenic heavy metal (Cd, Cu, Sb, Zn) emissions in the Soviet Union
The development of strategies and policies aiming at the reduction of environmental exposure to air pollution requires the assessment of historical emissions. Although anthropogenic emissions from the extended territory of the Soviet Union (SU) considerably influenced concentrations of heavy metals in the Northern Hemisphere, Pb is the only metal with long-term historical emission estimates for this region available, whereas for selected other metals only single values exist.
Effect of surface charge density on the affinity of oxide nanoparticles for the vapor–water interface
Using in-situ X-ray photoelectron spectroscopy at the vapor-water interface, the affinity of nanometer-sized silica colloids to adsorb at the interface is shown to depend on colloid surface charge density. In aqueous suspensions at pH 10 corrected Debye-Hückel theory for surface complexation calculations predict that smaller silica colloids have increased negative surface charge density that originates from enhanced screening of deprotonated silanol groups by counterions in the condensed ion layer.
Chemistry: Ten things we need to know about ice and snow
Understanding the molecular behaviour of frozen water is essential for predicting the future of our planet, says Thorsten Bartels-Rausch.
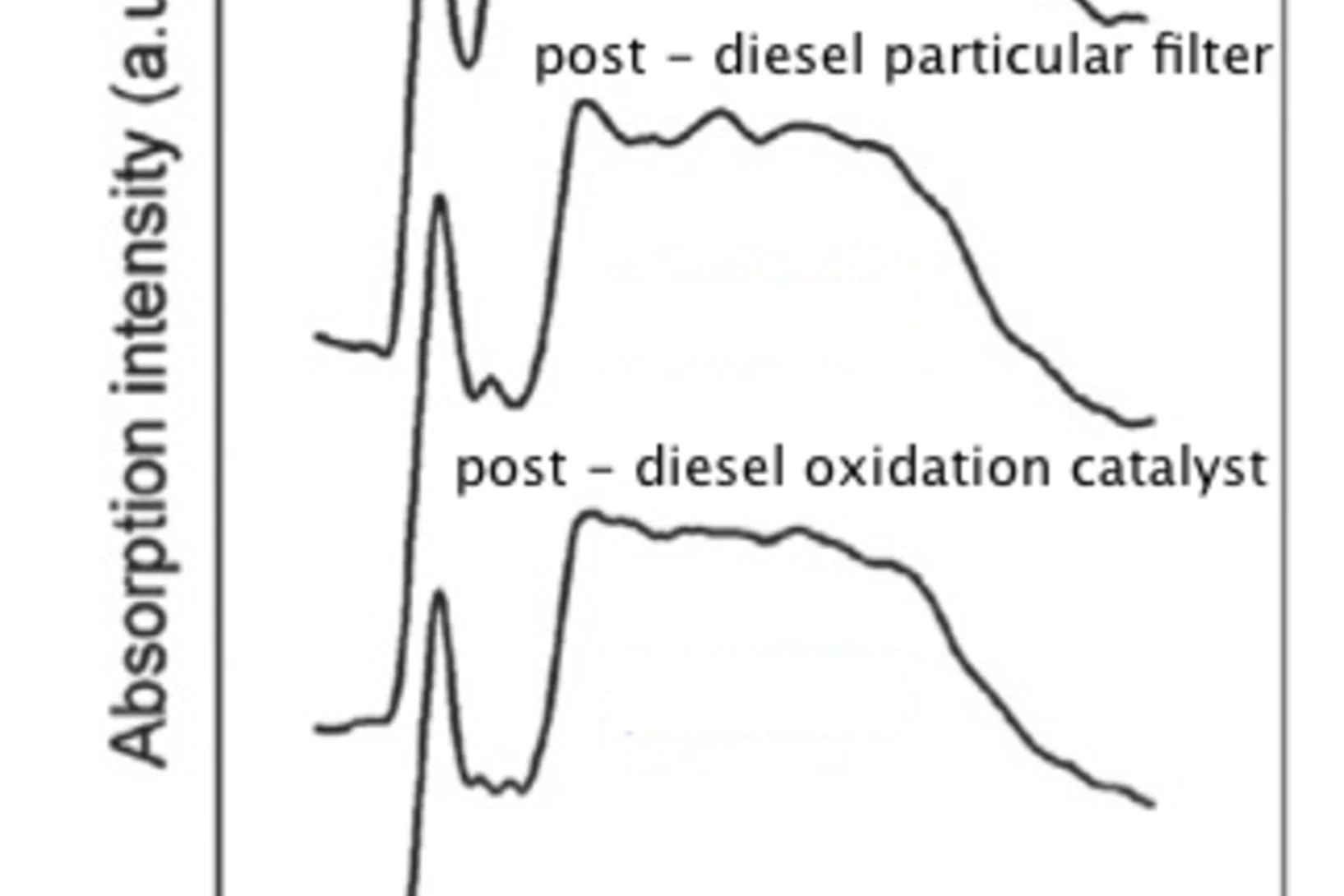
Variations in diesel soot reactivity along the exhaust after-treatment system, based on the morphology and nanostructure of primary soot particles
The reactivity of soot at different sites of the exhaust after-treatment system of a diesel engine (upstream and downstream of the diesel oxidation catalyst (DOC), downstream of the diesel particulate filter (DPF), as well as inside the DPF) was investigated on the basis of morphology and structure of primary soot particles by high resolution transmission electron microscopy (HRTEM). The results indicate that combustion-formed soot particles are susceptible to further transformations of their morphology within the exhaust system.
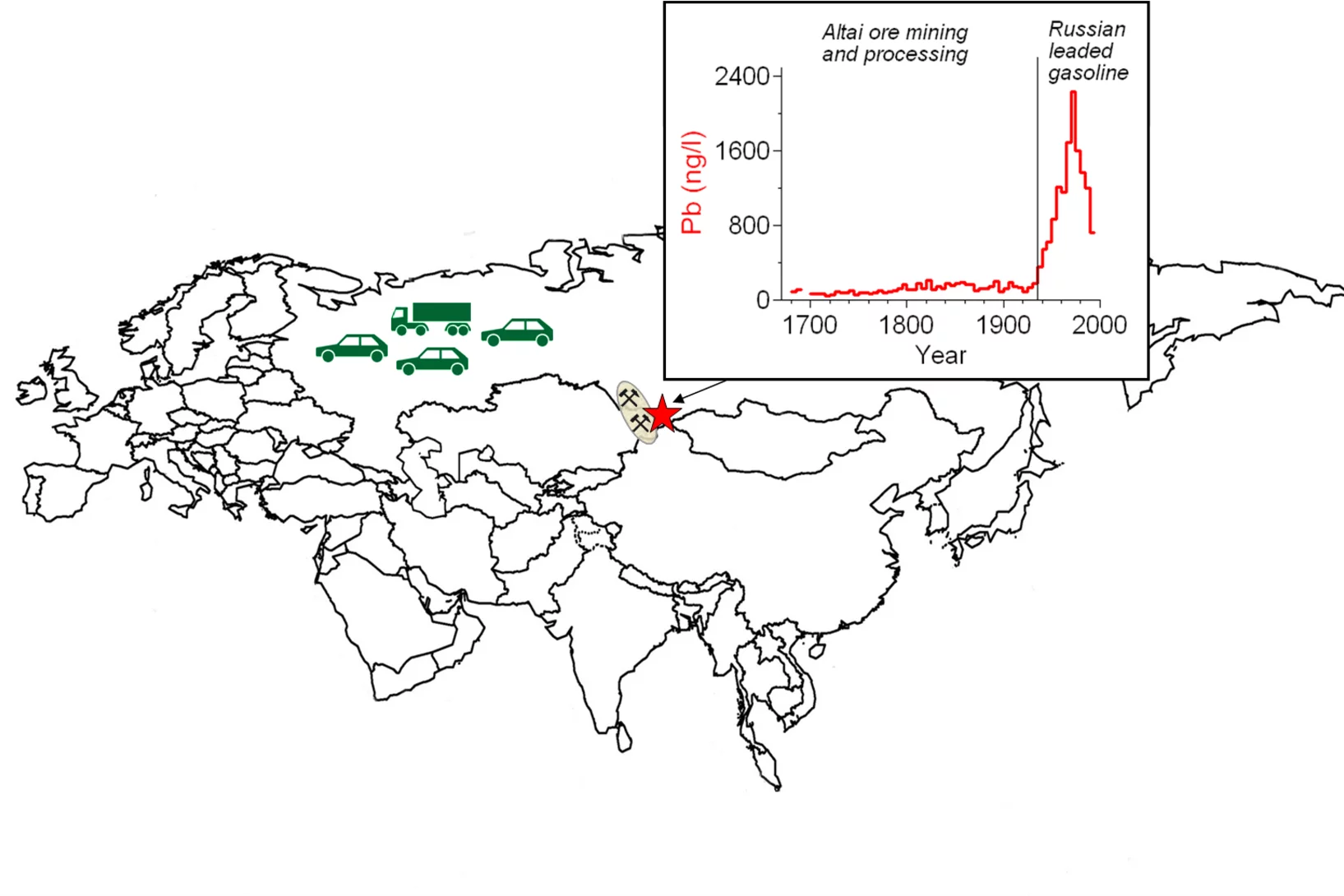
Three centuries of eastern european and Altai lead emissions recorded in a belukha ice core
Human activities have significantly altered atmospheric Pb concentrations and thus, its geochemical cycle, for thousands of years. Whereas historical Pb emissions from Western Europe, North America, and Asia are well documented, there is no equivalent data for Eastern Europe. Here, we present ice-core Pb concentrations for the period 1680à1995 from Belukha glacier in the Siberian Altai, assumed to be representative of emissions in Eastern Europe and the Altai.
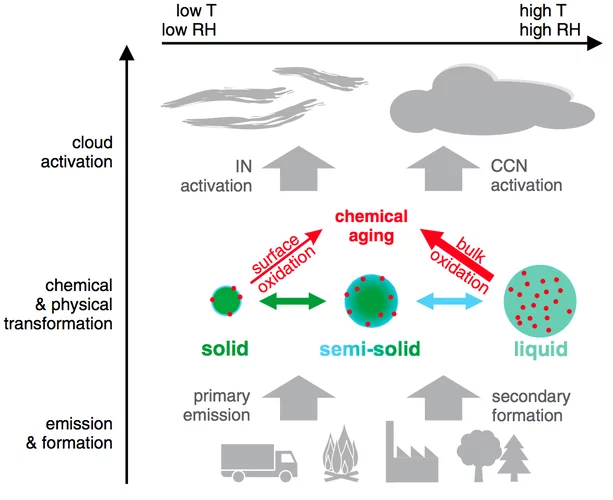
Gas uptake and chemical aging of semisolid organic aerosol particles
Organic substances can adopt an amorphous solid or semisolid state, influencing the rate of heterogeneous reactions and multiphase processes in atmospheric aerosols. Here we demonstrate how molecular diffusion in the condensed phase affects the gas uptake and chemical transformation of semisolid organic particles. Flow tube experiments show that the ozone uptake and oxidative aging of amorphous protein is kinetically limited by bulk diffusion.
The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles
The heterogeneous reactions of ozone with aerosol particles are of central importance to air quality. They are studied extensively, but the molecular mechanisms and kinetics remain unresolved. Based on new experimental data and calculations, we show that long-lived reactive oxygen intermediates (ROIs) are formed. The chemical lifetime of these intermediates exceeds 100 seconds, which is much longer than the surface residence time of molecular ozone (~ ns).
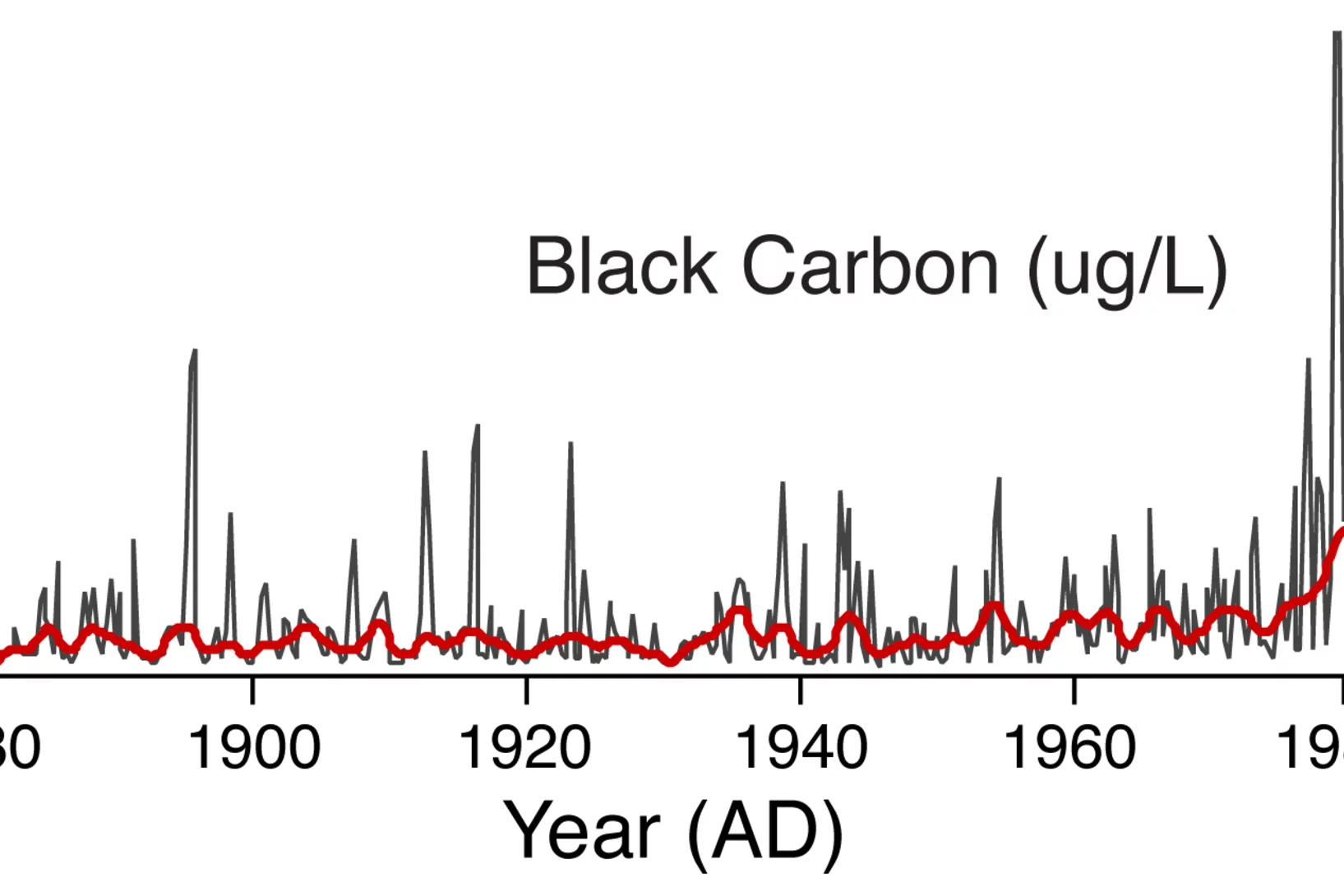
Recent increase in black carbon concentrations from a Mt. Everest ice core spanning 1860–2000 AD
A Mt. Everest ice core spanning 1860à2000 AD and analyzed at high resolution for black carbon (BC) using a Single Particle Soot Photometer demonstrates strong seasonality, with peak concentrations during the winter‐spring, and low concentrations during the summer monsoon season. BC concentrations from 1975à2000 relative to 1860à1975 have increased approximately threefold, indicating that BC from anthropogenic sources is being transported to high elevation regions of the Himalaya.
The nature of nitrate at the ice surface
Trace contaminants such as strong acids have been suggested to affect the thickness of the quasi-liquid layer at the ice/air interface, which is at the heart of heterogeneous chemical reactions between snowpacks or cirrus clouds and the surrounding air. We used X-ray photoelectron spectroscopy (XPS) and electron yield near edge X-ray absorption fine structure (NEXAFS) spectroscopy at the Advanced Light Source (ALS) to probe the ice surface in the presence of HNO3 at 230 K.
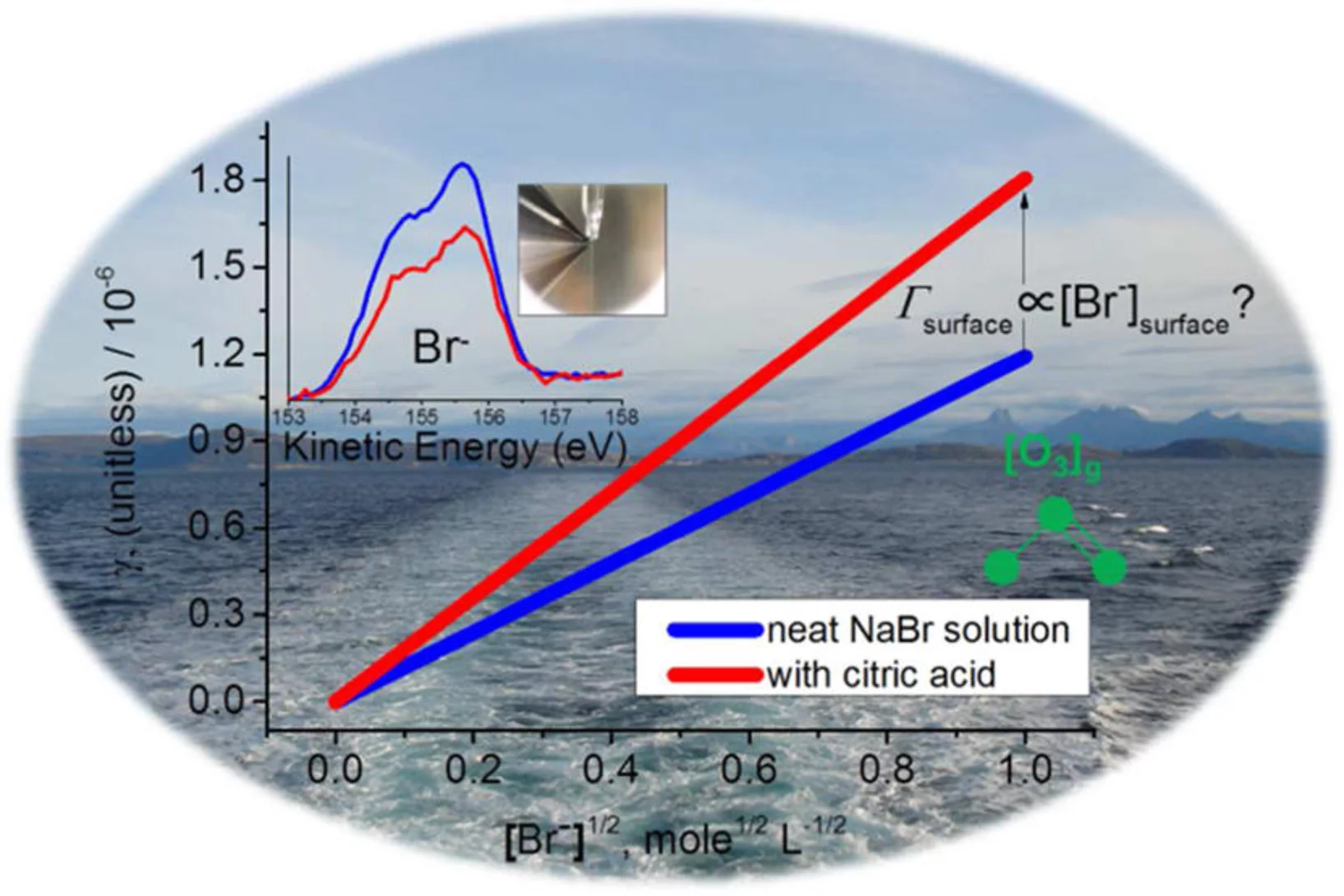
The competition between organics and bromide at the aqueous solution – air interface as seen from ozone uptake kinetics and X-ray photoelectron spectroscopy
A more detailed understanding of the heterogeneous chemistry of halogenated species in the marine boundary layer is required. Here, we studied the reaction of ozone (O3) with NaBr solutions in presence and absence of citric acid (C6H8O7) under ambient conditions. Citric acid is used as a proxy for oxidized organic material present at the ocean surface or in sea spray aerosol.