Investigating the Role of Strain toward the Oxygen Reduction Activity on Model Thin Film Pt Catalysts
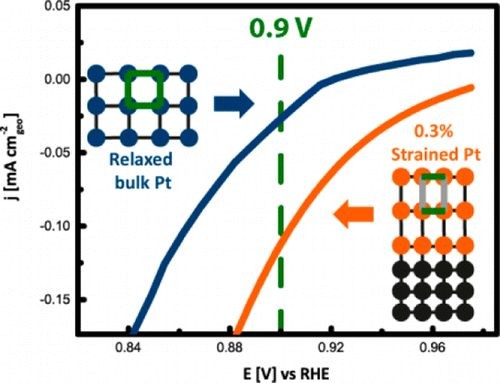
Environmentally friendly energy conversion devices such as fuel cells are becoming more and more attractive. However, major impediments to large-scale application still arise on the material side, related to the cost and poor performance of the cathode catalyst. State-of-the-art electrocatalysts are all Pt-based materials, suffering from poor electrochemical oxygen reduction kinetics. Tuning the interatomic distance of Pt atoms represents a promising strategy for reducing the strength of adsorption of oxygenated species to the Pt surface and thus improving the kinetics. In this context, model Pt electrocatalysts of strain-induced varied interatomic spacing were fabricated and tested. Strained Pt films with high crystalline quality can be obtained via epitaxial growth on appropriate single-crystal substrates like (111) SrTiO3, which have lattice parameters different from those of Pt using pulsed laser deposition. Through a proper selection of deposition parameters, the extent of strain in the Pt films can be controlled. This study shows that strain significantly modifies the electrochemical surface properties. In particular, cyclic voltammetry and CO oxidation experiments provide valuable insights into the effect of strain on the adsorption properties of spectator species (e.g., OHad and bisulfates) relevant for oxygen reduction reaction (ORR) kinetics. Furthermore, the strained Pt films exhibit a remarkably higher oxidation reduction reaction activity compared to that of the fully relaxed bulk structure as obtained from ORR polarization curves. This research highlights the importance of proper model systems with defined physical properties to establish design principles for better-performing catalysts.